Authors / metadata
DOI: 10.36205/ trocar3.2022002
Abstract
In patients with endometriosis, there are 2 types of endometria: the ectopic endometrium which defines the disease; and the eutopic endometrium, the one normally located inside the uterine cavity. Today, there is consistent data about both tissues. The existence of interferences and mutual impacts between the two has always been a matter of interest for researchers. In this article, we are addressing the impact of endometriosis on the eutopic endometrium as well as the role of hysteroscopy in diagnosing the resulting changes at an early stage. Chronic enhanced inflammation as well as progesterone resistance are changing the paradigm of the disease, previously restricted to hyperestrogenia. This results in a dysfunctional inflammatory endometrium, with enhanced vascularity and proliferative trends, ranging from endometrial polyps to carcinoma. Hysteroscopy plays a capital role in diagnosing the different stages of this impact. Practitioners need to increase awareness of this issue by giving closer attention to the intrauterine mucosa. Although more studies are necessary, eutopic endometrium of patients with endometriosis already appears as important to monitor on the long term. To our best knowledge, this is the first paper to directly address this problematic issue.
Introduction
The impact of endometriosis on eutopic endometrium is one of the most overlooked facets of the disease, receiving limited attention from researchers and practitioners. In our usual general thinking, it is mostly thought of as a consequence of impaired ovarian functions. In fact, endometriosis is well known to cause ovulatory disorders. The latter do ultimately impact the eutopic endometrium, as it is a hormone responsive mucosa. The resulting hyperestregenia resulting from chronic dysovuluation is thought to alter the eutopic endometrium.
However, endometriosis, as a chronic inflammatory disease par excellence, does affect the eutopic endometrium, even in normally ovulating patients. When practiced in a thoughtful way by a practitioner aware of these changes, hysteroscopy allows to diagnose this impact at different stages.
The changes of the eutopic endometrium in patients with endometiosis as compared to women without endometriosis: Different parameters have been reported to be impaired in the eutopic endometrium of patients with endometriosis: higher density of nerve fibers; quantitative and qualitative differences in expression of Epidermal growth factor system; altered proteome displaying evident trends of dysregulation, to mention just a few (1-3). To date, researchers aim to set a list of characteristic changes of the mucosa as biomarkers for the diagnosis of endometriosis via an endometrial sampling, an interesting diagnostic approach which still remains to be defined (4).
Researchers agree that the ensemble of the fundamental abnormal changes within the eutopic endometrium of women with endometriosis globally shows tendency towards enhanced proliferation and angiogenesis (5). The main anomalies can be summarized in two essential categories: progesterone resistance and impaired endometrial inflammation.
Progesterone resistance was a very interesting discovery. The eutopic endometrium in women with endometriosis does not properly express the hormone receptors, even in normally functioning ovaries. This aspect is changing the paradigm of the disease from an estrogen-dependent condition to a progesterone-resistance problem (6,7). Additionally, endometriosis being a major inflammatory disease, does also affect the patient’s eutopic endometrium. Among the impaired inflammatory parameters which have already been described within the mucosa: over-expression of pro-inflammatory cytokines; auto-antibodies against endometrial cells as well as a different behavior of some endometrial immune cells with a manifest trend toward pro-inflammatory properties, compared to women without the disease (8).
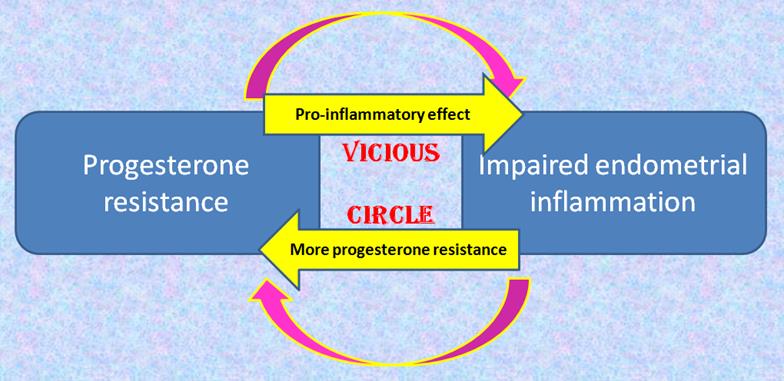
Another interesting fact is the close interrelation between progesterone resistance and impaired endometrial inflammation [fig 1]. On the one hand, inflammation is already known to cause progesterone resistance (9). On the other hand, the anti-inflammatory properties of the latter hormone have been demonstrated in physiology, as it suppresses production and action of proinflammatory markers and cells (7). Progesterone withdrawal at the end of the menstrual cycle allows the immune cells to resume their cytotoxic effect, which partly contributes to the shedding of the mucosa and the onset of menses (10,11) Progesterone resistance negatively interferes with this physiological process and results in a vicious circle. On the one hand, a pro-inflammatory environment facilitated by progesterone resistance, and subsequently aggravating it.
This explains why chronic endometritis (CE) was reported to be highly associated with endometriosis (12). However, CE, as currently defined, is a problematic entity. In fact, it is consensually accepted as a chronic inflammation within the endometrium, caused only by germs and to be treated exclusively with antibiotics (13-14). This is a definition we strongly criticize for many basic reasons. On the one hand, the only randomized control trial assessing the impact of antibiotics did not support their effectiveness in terms of reproduction (15). On the other hand, chronic inflammation is a capital component of the normal endometrium (8). In fact, the normal menstrual cycle has always offered an extraordinary template for physiologists and immunologists to study inflammation, as it involves cyclical injury, bleeding, pain, pro-resolving self-limiting healing, regeneration of the mucosa, recruitment of inflammatory cells in the secretory phase whose cytotoxic effect is kept inhibited by progesterone (11). After the drop of the hormone at the end of the cycle, the immune cells resume their cytotoxic activity and contribute to the shedding of the mucosa (10). Consequently, chronic inflammation is a normal component of the endometrium, and only becomes problematic when impaired, when the inflammatory balance is broken in favor of the pro-inflammatory mechanisms over the anti-inflammatory ones (8). Another basic fact in immunology is not only germs can trigger an inflammatory response. Endometriosis is a perfect demonstration of an inflammatory disease, not caused by germs, but by the presence of an inflammatory tissue in ectopic locations. It provides another illustration of the concept we defend, termed “impaired inflammatory state of the endometrium” (IISE), as opposed to CE (8). In case of endometriosis, chronic perturbed inflammation results in a chronic IISE (C-IISE) The consequences of C-IISE and chronic state of progesterone resistance on eutopic endometrium:
Although the most frequently mentioned consequences are infertility, implantation failure and miscarriage, the long term complications can be much more dramatic because progesterone resistance and C-IISE provide the optimal ground for abnormal proliferation, with areas of non shed endometrium, ultimately resulting in an endometrial cancer (8). Less severe intermediate proliferative conditions are more likely to occur in this context: endometrial polyps and hyperplasia (8,16).
In fact, many studies have provided epidemiological evidence that endometrial carcinogenesis could be promoted by an inflammatory milieu (17-19). It is no wonder that endometriosis is associated with a higher risk of endometrial cancer (20). A nationwide population based Taiwanese cohort study clearly showed increased risk of endometrial cancer in the later life of patients with endometriosis (21). Nevertheless, larger investigations are needed.
Practical implications: hysteroscopy in patients with endometriosis
Growing awareness of the changes endometriosis causes in the eutopic endometrium leads to a more thoughtful practice, with better knowledge regarding the anomalies to be careful about.
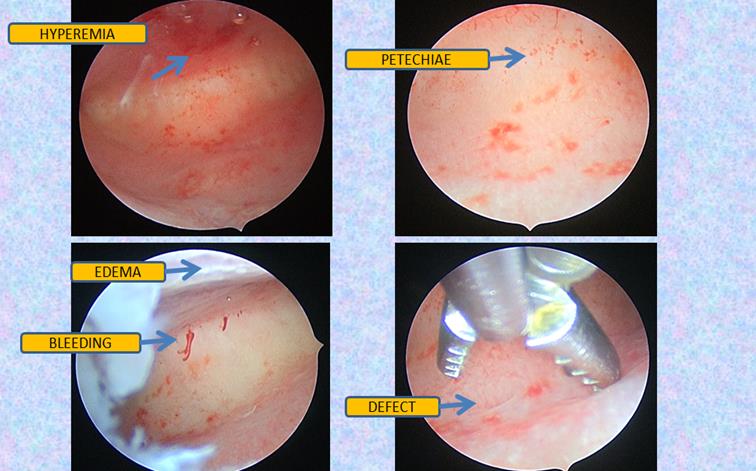
Progesterone resistance and impaired inflammation are the core problem, and still, two sides of a single coin. IISE is the very first change that occurs in the eutopic endometrium. However, even expert hysteroscopic surgeons might give limited attention to inflammatory mucosa and simplistically conclude “normal hysteroscopy” when no synechia or “overgrowths” are visible inside the cavity. In Figure 2, we report the hysteroscopic iconography of a 30 years old patient with a medical history of endometriosis, 4 failed IVF and 3 hysteroscopies performed by expert hysteroscopists, all concluding “normal hysteroscopy” whereas redness was noticeable in the images printed with the descriptive report. When performing her last hysteroscopy required by the new assisted reproductive technology center, subtle inflammatory patterns were identified: bleeding, redness, petechiae, irregular proliferation and focal defects. A targeted biopsy, performed within a complex inflammatory lesion comprising defect and hyperemia, allowed the diagnosis of dysfunctional endometrium with plasma cells to be posed. The patient received antibiotics, anti-inflammatory drugs and GnRH before a new IVF, followed by low dose aspirin. This resulted in a successful twin pregnancy with live births.
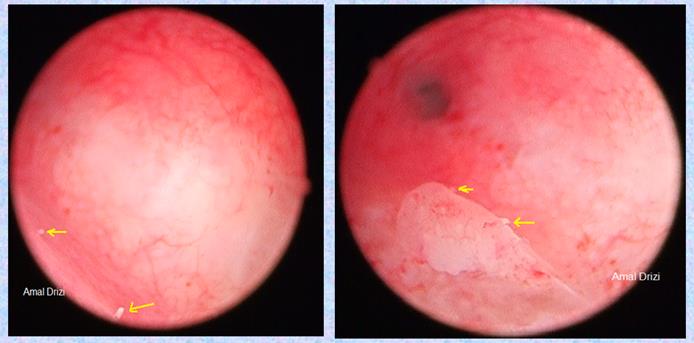
Another typical sign of chronic IISE are micropolyps. Very interestingly, studies revealed their rich content in inflammatory cells, among which plasma cells (22). The biopsy should optimally target the areas containing these inflammatory lesions (Fig 3).
In a recent article was proposed a simplified protocol of hysteroscopic signs suggestive of dysfunctional inflammatory endometrium, founded on a histopathological basis (23). Among the new hysteroscopic criteria are the irregular interglandular spacing, and focal areas of corrugated surface due to irregular thickness (Fig 3).
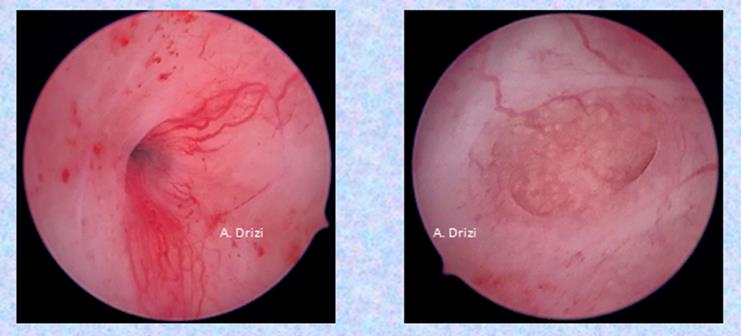
Another consequence of persistent IISE with progesterone resistance is enhanced vascularity (Fig 4) and proliferative disorders, ranging from polyps to carcinoma.
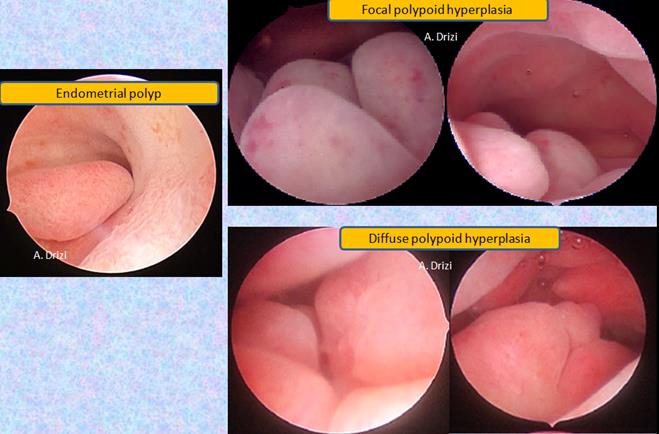
In patients with endometriosis, particular attention has to be paid to the presence of endometrial polyps (16). The context of chronic inflammatory environment and progesterone resistance caused by endometriosis theoretically benefits volume, number and recurrence of polyps, ultimately resulting in a polypoid hyperplasia. The latter is either focal or diffuse, with or without atypia (Fig 5). All the figures shared are from patients with documented endometriosis.
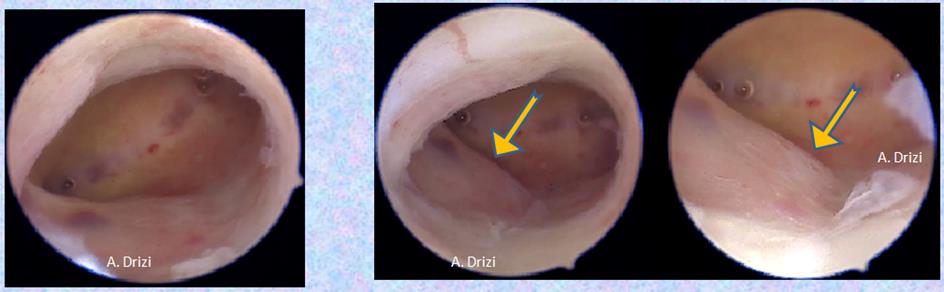
In our practice of hysteroscopy, it is important to remember that hyperplasia does not always present in massive polyploid patterns. It could develop in a more subtle way, requiring more attention. In figure 6, a focal hyperplasia is diagnosed in a patient with endometriosis and adenomyosis, initially presenting as a hardly visible bump in the posterior lateral wall. Closer view reveals the thickening of the endometrium, which was sampled. Anatompathological examination confirmed adenomyosis, hyperplasia without atypia and IISE.
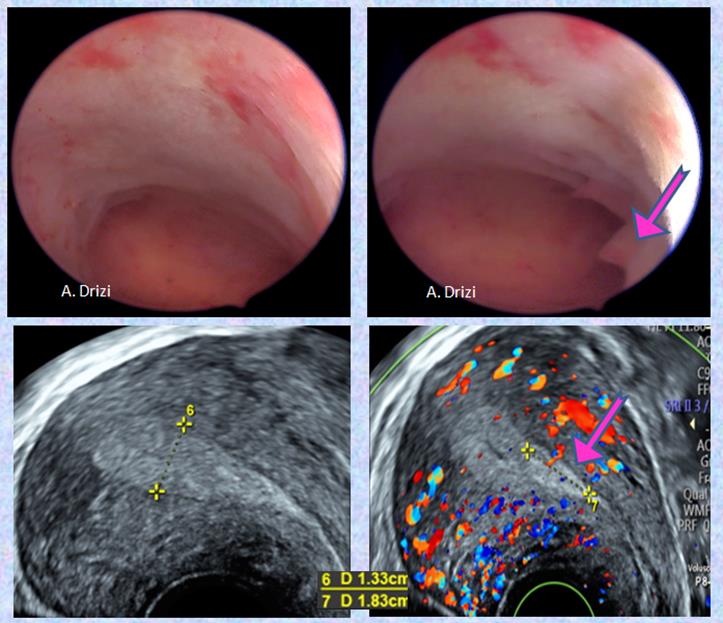
Moreover, when hyperplasia is uniformly diffuse, it leads to more difficulties at hysteroscopy. The role of ultrasound comes in handy in these situations, demonstrating a uniformly abnormally thickened endometrium for the phase of the menstrual cycle (Fig 7).
The chronic progesterone resistance state which characterizes the eutopic endometrium of patients with endometriosis, theoretically explains the issues of slow response to progesterone therapy and recurrence of hyperplasia we experience in our practice with this condition. This needs to be properly assessed in large studies and in the meantime, taken into consideration for the patients’ benefit.
Growing awareness about the various changes in the eutopic endometrium of women with endometriosis highlights the importance of monitoring the endometrium of these patients at least with ultrasound, and when indicated, with hysteroscopy. Our paper stresses the importance of a more thoughtful practice of hysteroscopy when indicated in these patients. However, although the eutopic endometrium of patients with endometriosis already appears important to monitor, more studies are necessary on different levels. On the one hand, the prevalence of the above-mentioned changes need to be more properly assessed in endometriosis patients as compared to women without endometriosis. On the other hand, the correlations between hysteroscopic images and histopathology need to be seriously developed. Unfortunately, few studies are available in this area, as the practice of diagnostic hysteroscopy faces serious issues in terms of standardization, especially in terms of examination and sampling techniques, explaining an extremely heterogeneous practice in the different parts of the world. Blind sampling continues to be widely performed, even by hysteroscopists sometimes. This is strongly impacting research in the negative way.
Other obstacles which are seriously hindering hysteroscopy are the higher cost as well as the lack of accessibility to this technology in many parts of the world. However, the major issue from our perspective is the limited interest of hysteroscopists themselves in developing diagnostic hysteroscopy as operative hysteroscopy continues to draw almost all the attention. Growing awareness of endometrial physiology and pathology needs to be a prerequisite for this practice, not only for hysteroscopists but for pathologists as well.
Conclusion
Because of the significant impact of endometriosis on eutopic endometrium, hysteroscopists need to grow awareness of the various changes caused within the mucosa. Endometriosis is documented to be significantly associated with IISE, progesterone resistance, endometrial polyps and carcinoma. The eutopic endometrium of women with endometriosis needs to be added to the list of the parameters to monitor in the long term by ultrasound, and in case of suspected pathology, by hysteroscopy. For this, further studies are necessary.
References
Fig 1: The main changes in the eutopic endometrium of patients with endometriosis: vicious circle
Fig 2: Subtle signs of IISE in a 30 years old patient with a history of 4 failed IVFs
Fig 3: Micropolyps defining the targeted biopsy site
Fig 4: enhanced regular vascularity (adénomyosis)
Fig 5: polypoid endometrium. In the left: unique polyp with an irregularly thick surrounding endometrium; in the right: focal and diffuse polypoid hyperplasia without atypia
Figure 6: focal hyperplasia without atypia in a patient with endometriosis and adenomyosis
Fig 7: Uniformly diffuse hyperplasia with a focal polypoid thinckening (adenomyosis)