Authors / metadata
DOI: 10.36205/trocar1.2023006
Abstract
Reporting a normal endometrium during a diagnostic hysteroscopy needs to be based on objective criteria taking into consideration the histological, physiological and pathological features of the mucosa. The evaluation can be challenging because of the cyclically changing character of the mucosa, displaying different features depending on the subphase of the menstrual cycle.
In this article, an atlas-like overview from a purely diagnostic perspective of the main features of the endometrium is provided with the main educational objective of facilitating the interpretation of the hysteroscopic images of the endometrium in a way which is based on fundamentals. The targeted states are normal, dysfunctional/inflammatory, adenomyotic, polypoid, hyperplastic and malignant endometria. The general principles of sampling and writing the descriptive report are further discussed. The assessment of endometrial vascularization is excluded as it is the subject of a different article in this special edition.
Introduction
The endometrium is the mucosa lining the uterine cavity, on top of the myometrium. The first concern of a hysteroscopist during a diagnostic procedure is to distinguish normal from abnormal, as this separation results in medical-legal consequences. Defining the normal endometrium is relatively complex as it is a cyclically-changing mucosa, with different aspects depending on the phase, and even at times on the subphase of the menstrual cycle. Consequently, no pertinent examination can be conducted in the ignorance of the basic sciences related to endometrial histology and by extension, physiology and pathology.
In this article presented in the form of an atlas, the main objective is to provide an educational simplified review of the endometrium in its different patterns: normal, dysfunctional, inflammatory, polypoid, hyperplastic and malignant, taking into consideration the available evidence of the literature.
Normal endometrium – a cyclically changing mucosa
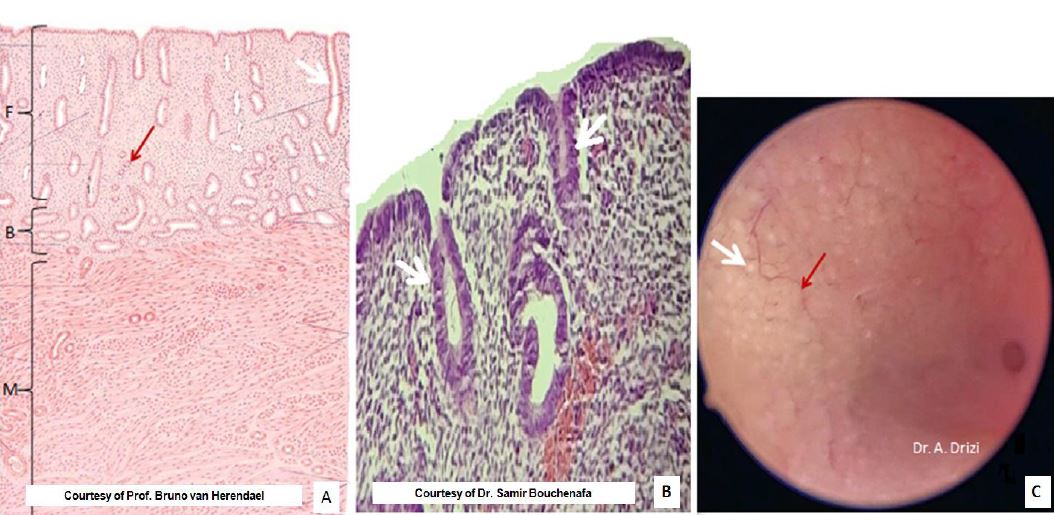
Regardless of the phase of the menstrual cycle, the global architecture of the endometrium remains the same, as it is composed of a surface epithelium which folds into perpendicular glands invaginating into the underlying stoma (1). The latter supports the regularly spaced endometrial glands and consists of a connective tissue where stromal cells and blood vessels fill in the interglandular spaces (fig 1). Moreover, the thickness of the mucosa contains two functionally distinct layers. The superficial one termed the functionalis is the one repeatedly shed during menses or, in case of pregnancy, the site of the embryo implantation. The basalis is the deeper layer whose role has always been postulated to permit regeneration of the shed functionalis. Recent studies revealed a horizontal network of gland rearrangements in the basalis in contrast to their vertical disposition in the functionalis (2) (fig 1). This complex horizontal network of mycelium-like interconnected glands was demonstrated to contain endometrial stem cells giving rise to the functionalis glands. This new disposition additionally explains the horizontal spread of stem cells thus allowing self-renewal of the entire endometrial glandular elements at an adjacent deficient region (2).
Because the single-layer surface epithelium is transparent, examination of the normal endometrium by hysteroscopy only reveals the pinkish underlying stroma with the perpendicular glands, appearing as evenly distributed white dots, surrounded by blood vessels in places (fig 1C).
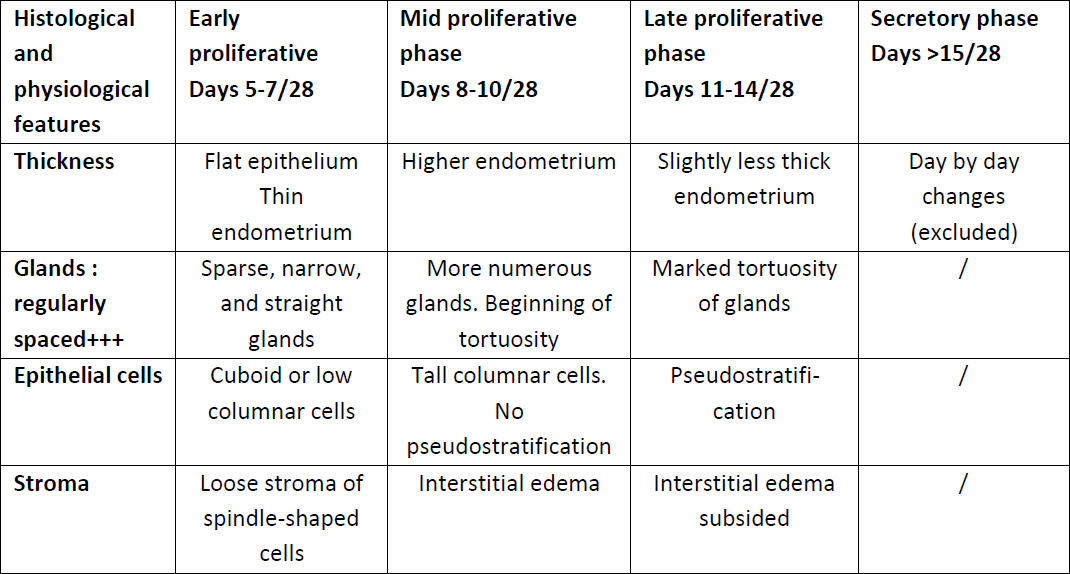
The above cited common features of the endometrium display changes throughout the menstrual cycle depending on the given phase (follicular and luteal) and sometimes even the subphase (early, mid and late proliferative or luteal) (1, 3). A description of the endometrium at the different phases of the menstrual cycle was published in a handout in early 2022 (4). Moreover, the main characteristics of the proliferative endometrium were described in details both histologically and hysteroscopically in a recent review article (3) and are summarized in Table 1.
Although hysteroscopy can also be performed during luteal phase, the proliferative phase was more targeted as it avoids the physiological thickening of the mucosa and increased cervical tonicity caused by the post-ovulatory hormonal environment (3).
Growing awareness of the normal and pathological features of the endometrium is of utmost importance for a proper hysteroscopic examination of the mucosa and also for a thoughtful interpretation of the pathologist’s report.
Among the common features of normal endometrium are the growing thickness of the mucosa throughout the menstrual cycle, the regular distribution of endometrial glands (figures 2, 3) and vessels. The stromal edema is a physiological finding during mid proliferative phase and luteal phase (1, 3, 5).
Although the endometrium is known to be a hormone-responsive mucosa, it is already well established in anatomopathology that the basalis and the isthmic mucosa are poorly responsive to hormones and hence cannot allow proper dating when sampled (6,7). Consequently, dating requires to avoid sampling within the isthmic region, hysteroscopically identifiable thanks to its anatomical landmarks which have been described by the anatomists in 1905, and at hysteroscopy in 2020 (8). The limits are the internal histological uterine os –commonly known as the internal os of the cervix– and the internal anatomical uterine os, located 6 to 10 mm above the first one (fig 4).
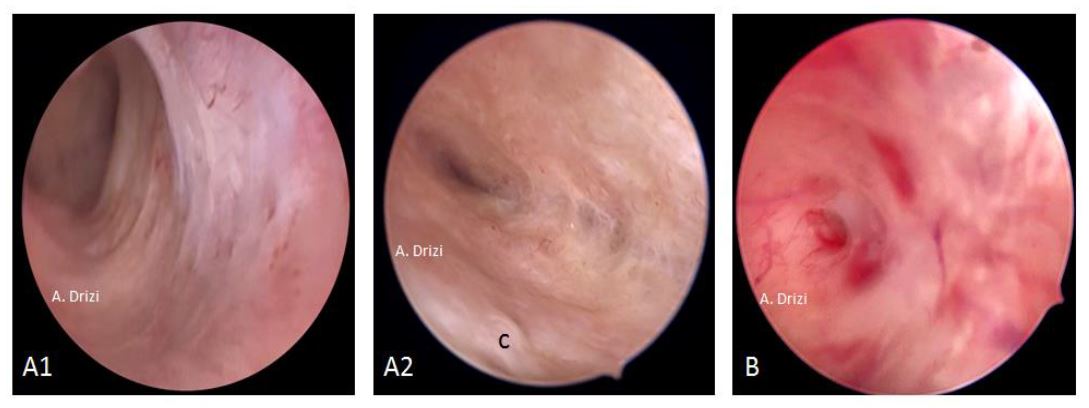
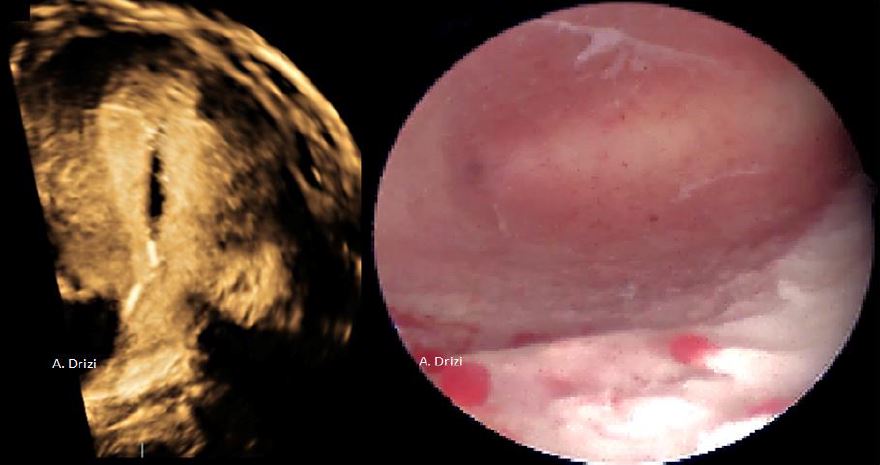
In post-menopausal patients and with the arrest of the cyclical hormonal activity, the endometrium enters into a chronic state of inactivity, consisting of a thin atrophic mucosa with scarce glands, sometimes dilated thus defining cystic atrophy (1, 6). The thinning of the mucosa can be so important that the marks of the underlying circular muscular fibers of the myometrium become visible (fig 5). Endometrial atrophy is also observed in breastfeeding women and in patients under chronic thinning hormone context (hyperprolactinemia, progesterone therapy, tamoxifen, to name just a few).
Dysfunctional inflammatory endometrium
The term dysfunctional etymologically designates perturbed functions of an otherwise organically normal structure. In the case of the endometrium, dysfunctional has long been exclusively associated with hormone imbalances, however there is a significant amount of literature linking hormone disorders to the broad spectrum of inflammation (3, 5, 9).
Unlike the common thinking in gynecology, inflammation is a normal component of the endometrial physiology and its impairment cannot be exclusively limited to microbial aggressions but to whatever disrupts homeostasis, such as allergens, oxidative factors, pollution, metabolic imbalances, adenomyosis, endometriosis as well as hormone imbalances to name just a few, resulting in an impaired inflammatory state of the endometrium (IISE) (10).
For the functionalis to shed during menses, the physiological endometrial inflammatory cells (mainly Natural Killer cells NKc) free their cytotoxic contents, along with the predecidual cells, under the effect of progesterone withdrawal at the end of the menstrual cycle. In fact, both progesterone and estrogens have already been established to have anti-inflammatory properties on the endometrium (10). The rise of estrogens in the beginning of the menstrual phase contributes to the self-limiting inflammatory destructive process caused by menses and subsequently, allows regeneration of the endometrium whose glands and stroma develop; and whose height progressively increases (11, 12). Consequently, not only ovarian impairment of sex hormones does impact these functions, but all the pro-inflammatory factors interfering with the endometrial inflammation as well, since excessive inflammation has already been demonstrated to impact the endometrium’s receptivity to normally-secreted ovarian hormones, thus resulting in a vicious circle (13). This explains how dysfunctional and inflammatory consist of two faces of a single coin, as they are closely interrelated (5, 13). This explains why, the presence of dysfunctional areas of endometrium in the entity chronic endometritis has been documented since the beginning of the past century and still to date (14).
These fundamentals need to be considered in our understanding and approach to dysfunctional inflammatory endometrium, to which the current definition of chronic endometritis does not do justice, hence the necessity to revise the current definitions or to propose more pertinent terminologies such as IISE or dysfunctional inflammatory endometrium (3, 10).
For a chronic IISE to cause clinical disorders (such as subfertility and recurrent miscarriage), stromal and/or glandular changes within the mucosa have already been presented necessary for the diagnosis by pathologists and physiologists, to such a point that their absence could ascertain the needlessness of a systematic search for plasma cells in the first place (7, 9). In fact, many pathologists agree that the diagnosis of chronic IISE should not rest on the unique finding of plasma cells in an endometrium that otherwise appears normal, because the background pattern is as important as the quantity of plasma cells (9, 14).
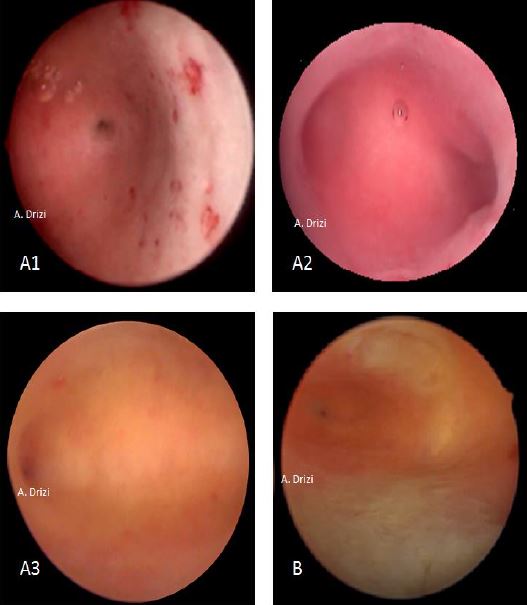
Among the hysteroscopic features of dysfunctional endometrium which can be correlated with histopathology are observed the commonly known signs for inflammation (15) (fig 6)as well as others which are overlooked andwhose importance has been highlighted in a recent review (3).
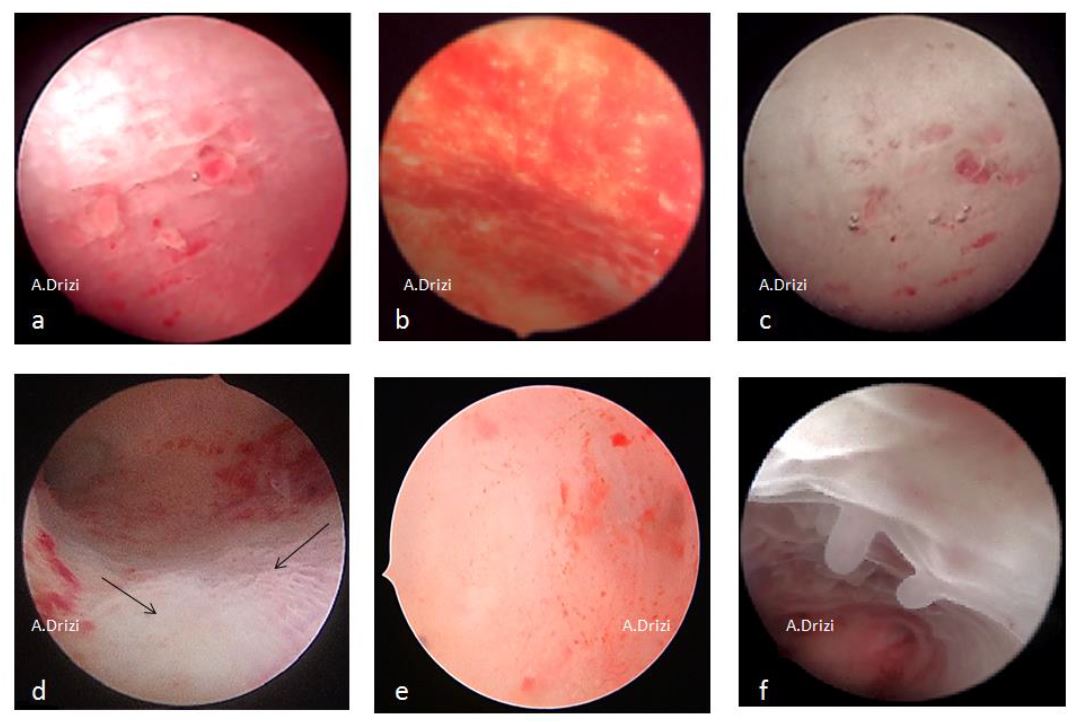
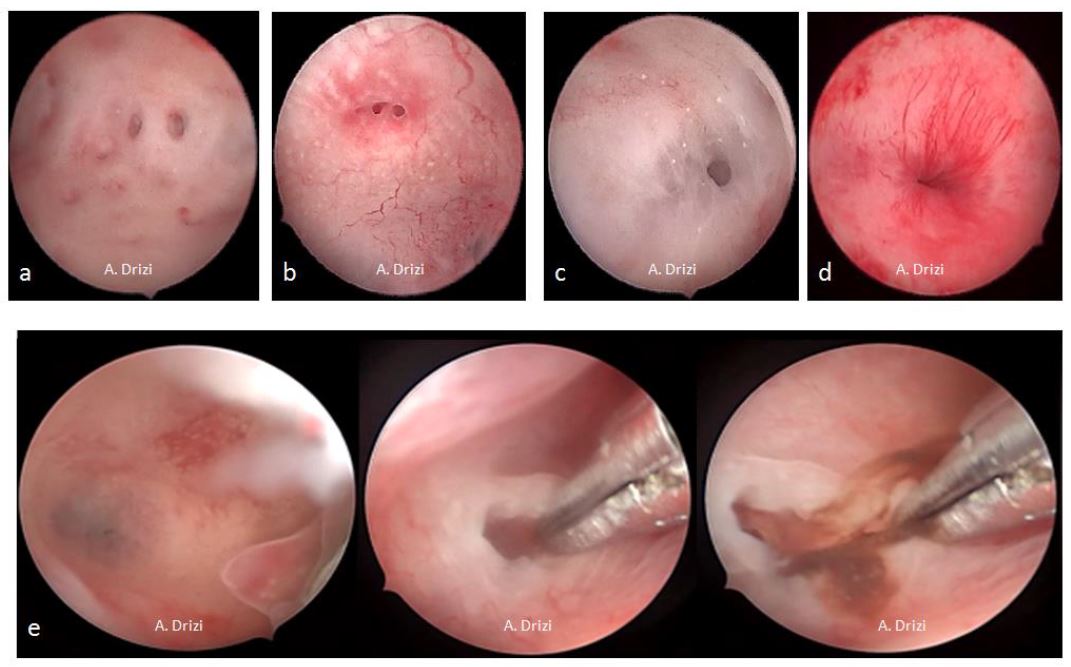
The classic signs of chronic inflammatory endometrium are micropolyps, strawberry aspect, stromal edema, hyperemia and hemorrhagic spots (fig 6). The specificity and sensitivity is lower for the last three signs, as these are also the classic cardinal signs for acute inflammation as well (3).
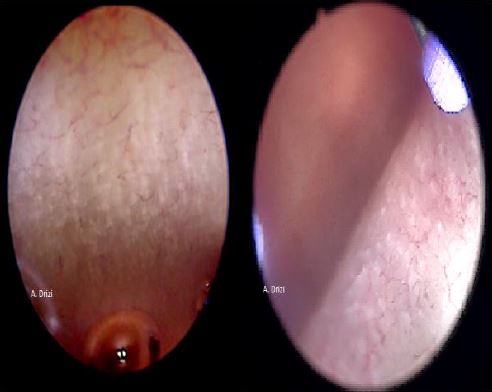
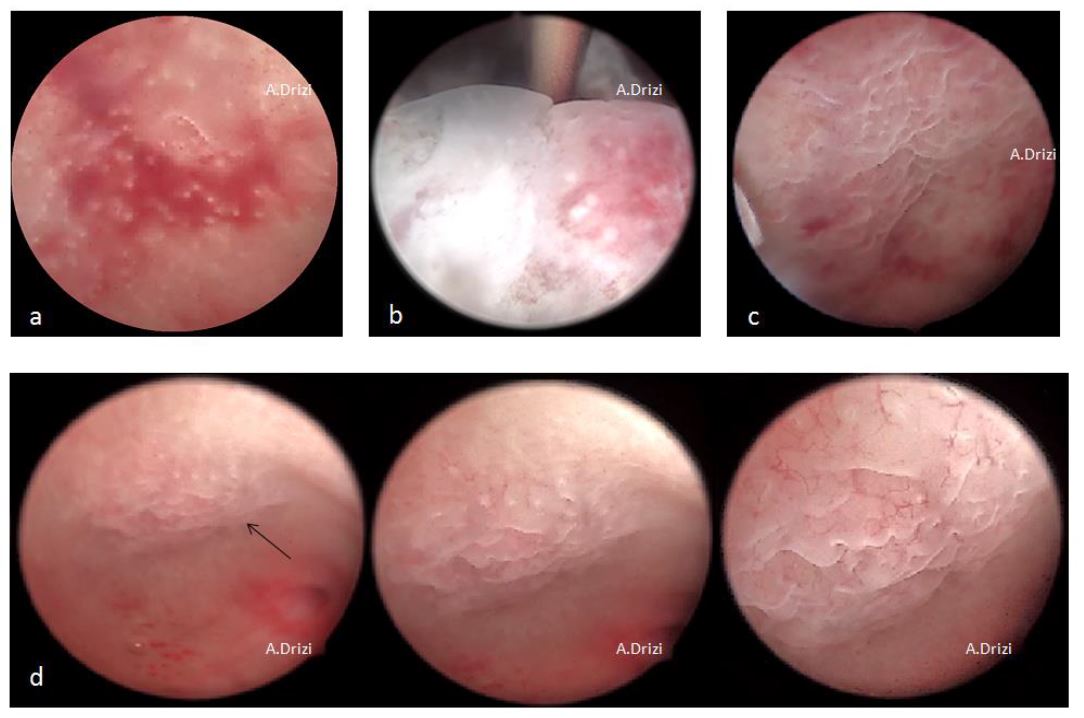
However, other hysteroscopic signs are overlooked although they are suggestive of a dysfunctional endometrium and hence are interesting to study as sampling sites (3). These additional signs are the irregular distribution of glands as well as the heterogeneous thickness of the endometrium, resulting in corrugated surfaces with embossed and debossed patterns (fig 7). In addition to stromal edema and endometrial hyperplasia, these aspects can be correlated with focal atrophic, deficient or resting endometrium; stromal breakdown and irregular proliferation, the latter being the dysfunctional disorder a stage below hyperplasia (3). Unevenly distributed glands are typically present in the strawberry aspect and in actual fact, all the above-mentioned signs can be associated with one another (3). In all cases, it is very important to be careful to these subtle aspects while performing a diagnostic hysteroscopy to optimize the sampling within the areas displaying these anomalies (fig 8).
Adenomyosis
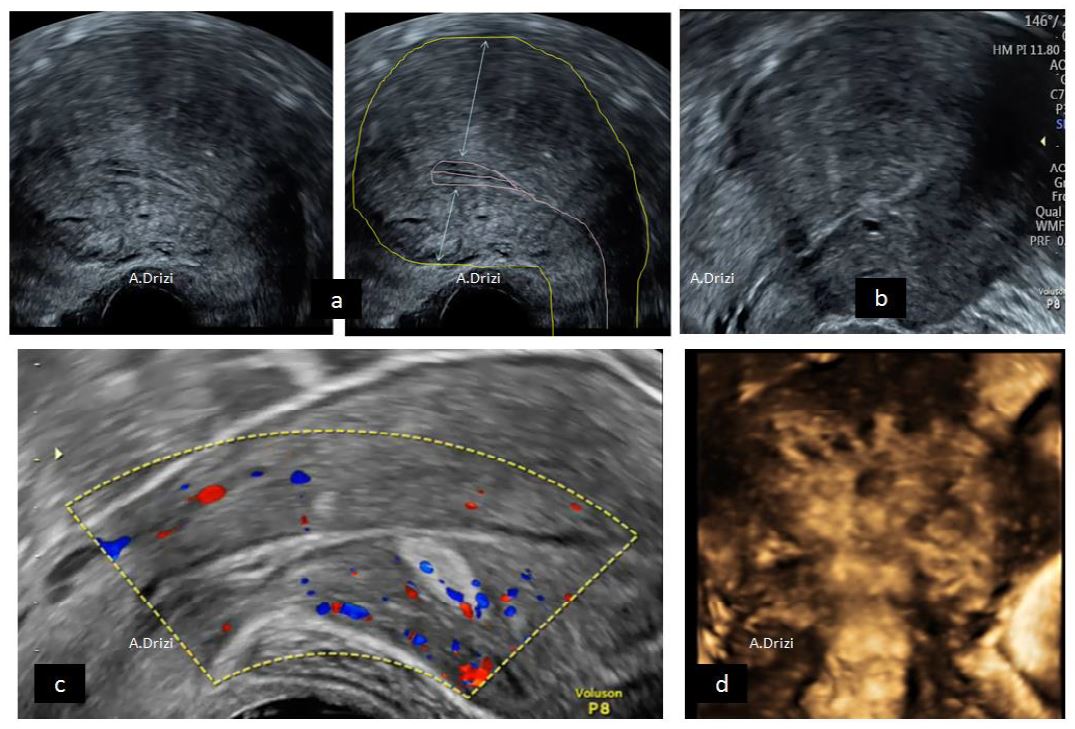
Among the chronic inflammatory diseases affecting women, adenomyosis is defined as the benign condition where active (hence inflammatory) endometrial glands and/or stroma are present within the myometrium. Among the common symptoms of the disease, heavy menstrual bleeding, secondary and/or aggravated dysmenorrhea, pelvic pain, subfertility as well as a higher risk of miscarriage/obstetrical complications have been reported (16). Imaging technology has provided consensual sonographic criteria suggestive of Adenomyosis by the Morphological Uterus Sonographic Assessment (MUSA) group (17), summarized in figure 9.
Although their pathognomonic character still remains to be proven, the following hysteroscopic signs are well accepted for adenomyosis and do more or so reflect the previously mentioned signs for dysfunctional inflammatory endometrium (fig 10): heterogeneous endometrial thickness with areas of focal defects (debossed patterns); submucosal hemorrhagic cysts displaying a dark blue or a chocolate brown appearance; fibrous cystic appearance of intrauterine lesions as well as hyper-vascularization (18, 19).
Endometrial polyps
An endometrial polyp (EP) is a focal overgrowth of endometrial glands and stroma classically thought of as a hormone-mediated condition in spite of the increasing evidence on the role of chronic IISE in generating it (10, 20). Although it is typically a benign anatomo-clinical entity, the prevalence of malignancy may range from 1 to 3%, particularly in post-menopausal patients (10). Many patients can be asymptomatic, however among the common symptoms of the disease are abnormal uterine bleeding (AUB), subfertility and pregnancy loss (21).
From a diagnostic perspective, ultrasound provides accurate definitions especially when combined with saline/gel infusion sonography (SIS) (22). The typical sonographic features of an EP are a hyperechoic image characterized by the presence of a unique feeding vascular pedicle (22). The number, size, implantation base and location can also be defined by 2D, 3D and SIS.
Consequently and as the diagnosis of EPs is accurately defined by imaging techniques, diagnostic hysteroscopy is to be scheduled only before operative hysteroscopy begins and aims at providing a confirmation as well as a more accurate description of the features which cannot be assessed by ultrasound such as surface color and micro vascularization. The most accepted classifications of EPs are related to their macroscopic aspects, which can be pedunculated or sessile, single or multiple, ranging from millimeters to centimeters, located in the fundus in more than 50% of cases (21) (fig 11).
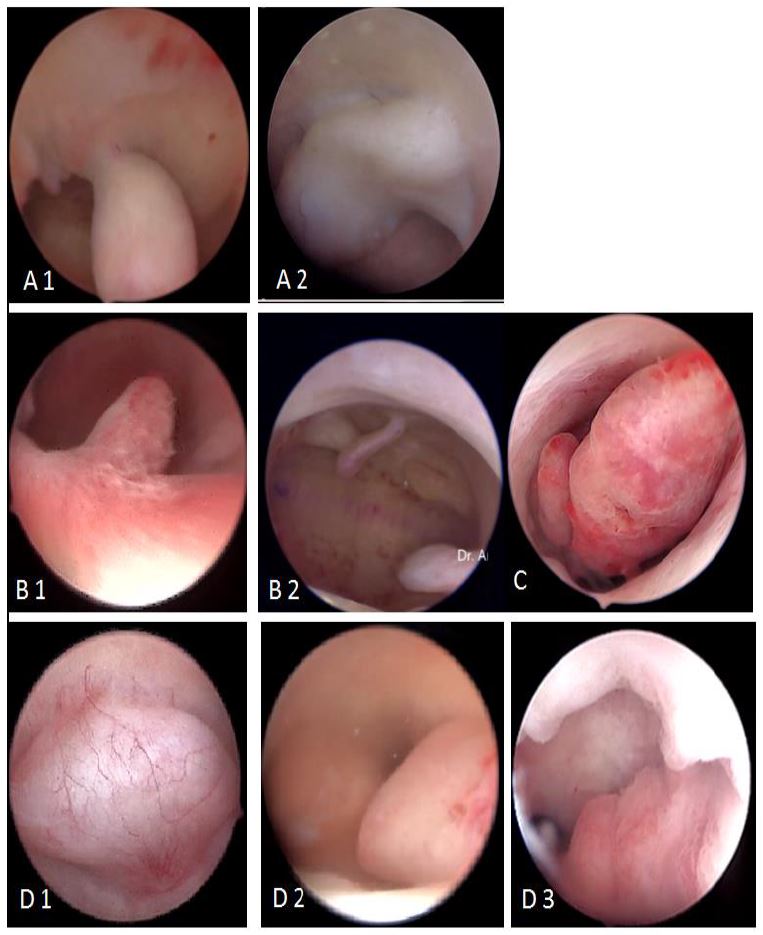
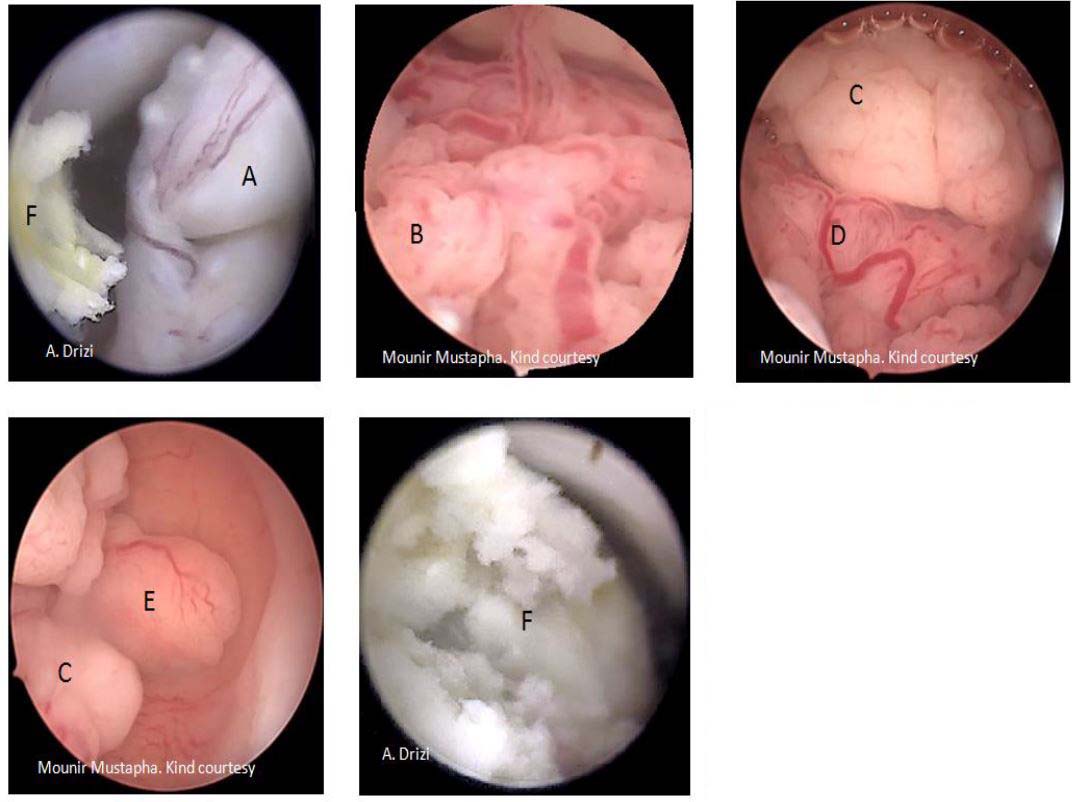
The histopathological classification separates pseudo polyps –which are physiologically and temporarily observed during luteal phase – from hyperplastic EPs which can contain atypia in places. The other classes are functional (disappear under hormone therapy); atrophic (especially in menopausal women) and adenomatous EPs. The latter additionally contain smooth and fibrous muscle tissue (21) (fig 12).
In addition to these histological types of EPs, others are encountered in our practice such as hemorrhagic and necrotic polyps displaying a blood-like color for the first and yellowish color for the second (fig 13). In all cases, the presence of a unique vascular pedicle is a sine qua none condition to define an EP at histopathology, which is commonly found at ultrasound but rarely seen in hysteroscopy (fig 13). However, the surface vascularity is always thin and regular.
The endometrium covering the EP has to be compared to the one lining the rest of the cavity. Other intrauterine pathology can be associated and if so will have to be reported.
Endometrial hyperplasia and malignancy
Both endometrial hyperplasia (EH) and malignancy (EM) are entities whose diagnosis requires anatomopathological examination. Hyperplasia is defined as an excessive growth of the endometrium with an increased gland to stroma ratio and an irregular glands distribution (23, 24). Since 2014, the World Health Organization (WHO) proposed a new classification of EH which was accepted by the International Society of Gynecological Pathologists, and which divided hyperplasia into two groups: benign hyperplasia and atypical hyperplasia/endometrial intraepithelial neoplasia (EIN) (25). The latter is characterized by the presence of cytological atypia in proliferating overcrowded, sometimes back-to-back glands. These changes are also present in endometrial carcinoma (EC) thus making the differential diagnosis of this malignancy in its lowest grade very challenging to distinguish from the most severe hyperplasias, to such a point that the gold standard for validation of the diagnosis of adenocarcinoma in histopathology is the presence of myometrial invasion by the hyperplastic atypical cells in hysterectomy specimen or deep biopsy (23, 24).
From a diagnostic perspective, the most frequently encountered symptom is AUB in both EH and EM. The International Endometrial Tumor Analysis (IETA) did set a consensus opinion regarding the pertinent sonographic criteria allowing the differential diagnosis between Ep, EH and EM (22) (fig 14). Still, the definitive diagnosis entirely belongs to histopathology.
In terms of diagnostic hysteroscopy, although there is to date no universal and reproducible morphological hysteroscopic definition of EH (26, 27), it is accepted that the condition appears as a diffuse or focal hypertrophy of the mucosa, displaying different patterns (fig 15). Depending on the severity of the thickening, the hyperplastic endometrium will adjust to the cavity’s volume by forming more or less big foldings mimicking endometrial polyps yet without a unique vascular pedicle but moderate vascularization. However, if the thickening is uniformly diffuse without any polypoid reinforcements, hysteroscopic diagnosis might be confusing whereas ultrasound allows accurate measurement of the mucosa’s thickness (fig 16). In these cases, paying attention to the subtle changes come in handy such as an irregular paler surface with edema, irregular glands distribution and increased micro vascularization.
The main differential diagnosis of EH without atypia is the late secretory phase and the dysfunctional inflammatory endometrium (3, 5). Atypical EH, also termed EIN, and EC however are likely to display similar patterns both hysteroscopically and histopathological (28). EM received higher attention from authors and different classifications have been proposed by different researchers to improve the hysteroscopic identification of the suspicious lesions to be visually biopsied. The neoplastic processes are categorized by their morphological external appearance. The terminology proposed by these classifications describe tumors as nodular (bulging), polypoid (thin pedicles), papillary (numerous dendritic projections), glomerular (polypoid with papillary-like component containing atypical neo-vessels covered by a thin layer of endometrial tissue) and cerebroid (nodular or polypoid with abnormal surface vessels) (29) (fig 17). Other terms were proposed by other authors such as ulcerated (endophytic) patterns and cotton-candy like (presence of necrosis) (29).
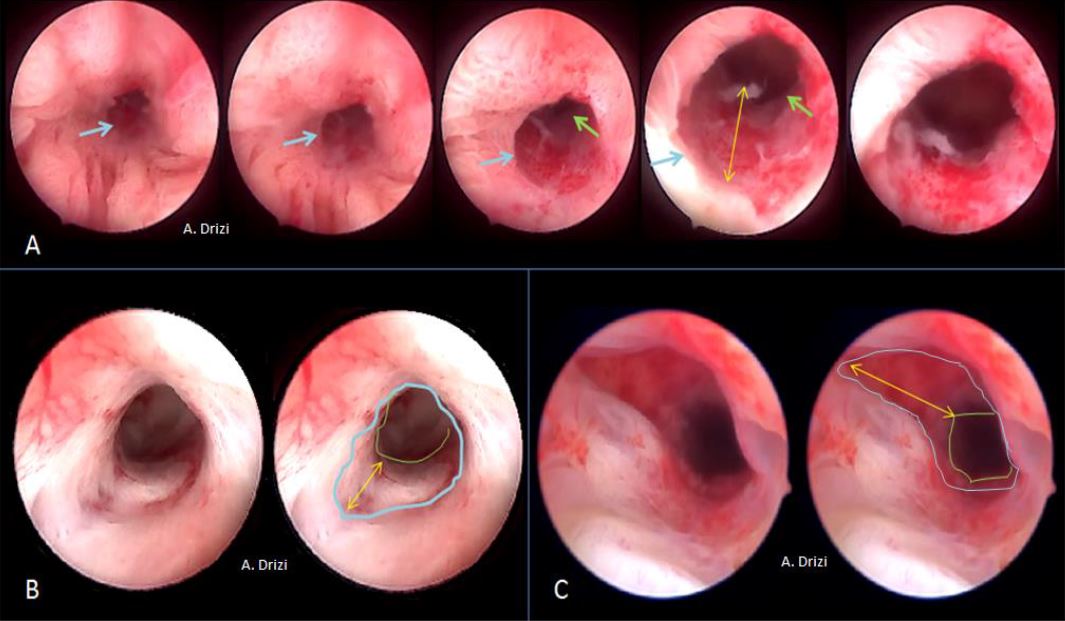
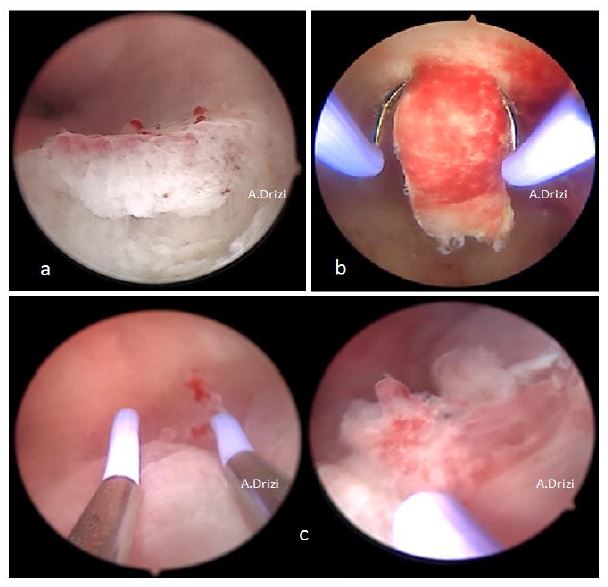
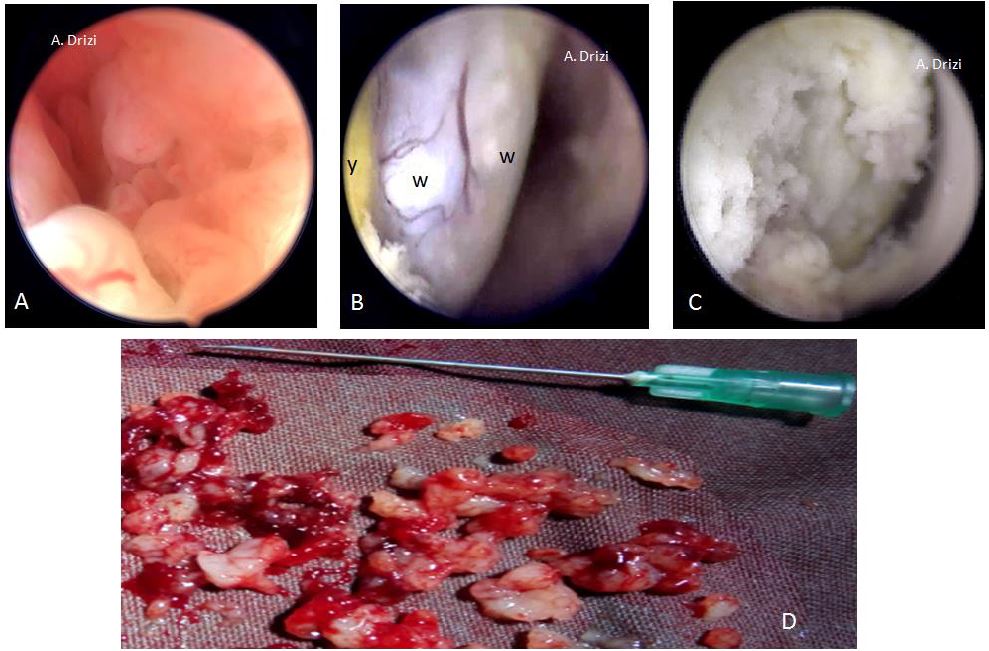
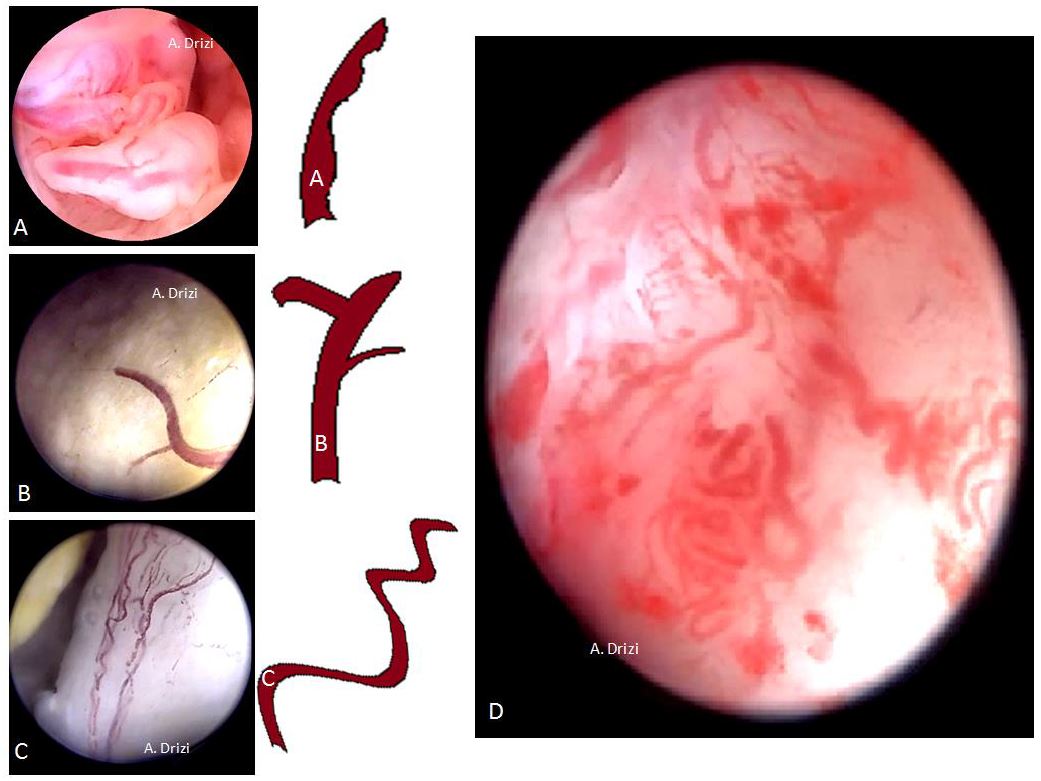
However, regardless of the terminology used in these different classifications, all seem to agree on the same morphologic criteria suggestive of atypical EH/EM and which are vascular, glandular and epithelial. Consequently and educationally speaking, it could be more interesting to focus on the analytic details of the tissue and vessels instead of the various classifications themselves. Those criteria indicate the site where the biopsy has to be performed, as all researchers confirmed their suspiciousness and use them to assess the risk in the above mentioned classifications. In addition to the lesion’s thickness (10 mm or greater) and its extension to the cervix, the morphologic epithelial criteria to be particularly careful about are illustrated in fig 18 and consist of an irregular surface (polypoid, endophytic or exophytic projections), heterogeneous colors (whitish-grayish without vessels on the surface: indicators of glandular back-to-back overcrowding; yellowish or whitish cotton-candy aspect as indicator of necrosis). The more the tissue is friable and hemorrhagic at hysteroscopic manipulation, the more suspicious (fig 18). And just as importantly, the characteristic suspicious vascular patterns present with irregular diameter, irregular ramifications, brutal interruptions and serpiginous trajectory forming loops at places (29,30) (fig 19).
General principles of sampling
The techniques of sampling are addressed in a separate article of this special edition. Nevertheless, the general principles related to tissue processing and examination by pathologists will be highlighted in order to grow awareness on the necessity of good practice.
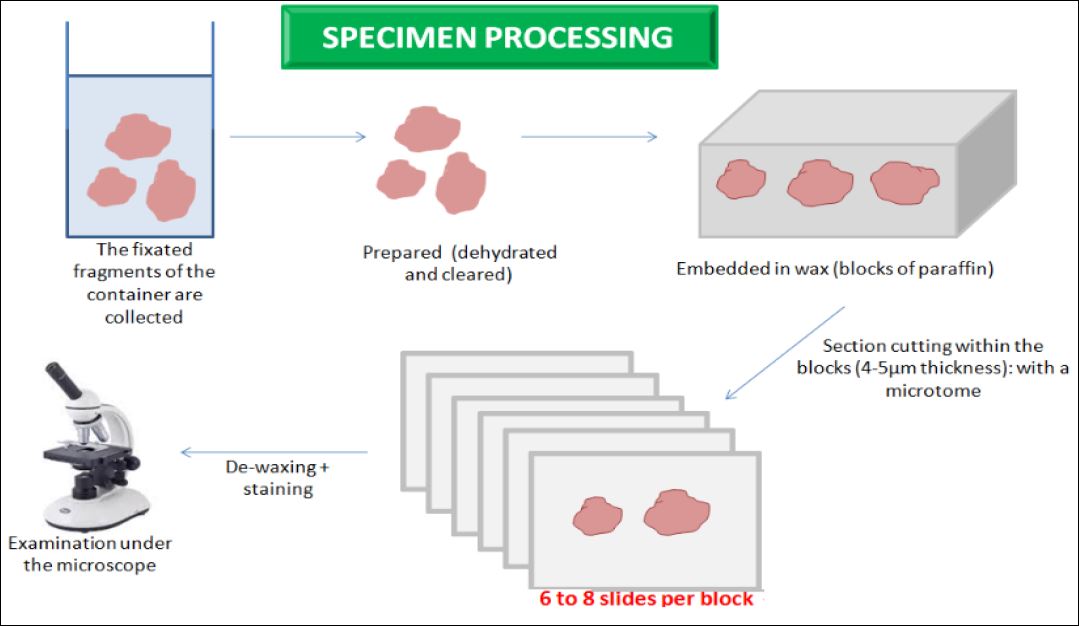
Because endometrial pathology is more commonly focal than diffuse (7), it is necessary to perform the sampling within the area displaying anomalies. The amount of tissue has to be sufficient and oriented so as to optimize the chances of a correct diagnosis by pathologists who need to receive proper information about all the cases. To ensure sufficient amount of tissue, different instruments can be used such as graspers, scissors, the loop of a min resectoscope or a tissue removal system. Because of the limited number of cuts pathologists universally perform (fig 20), it is always best to label and send in a separate container whatever lesion displaying suspicious or different aspects from the other lesions. To avoid further artifacts, the sample needs to be handled with gentleness, without compression –as it could create a false back to back distribution of glands– and always placed in a correct conservative milieu made of 15 volume equivalents of 10 % formalin per volume of tissue (31).
The descriptive report
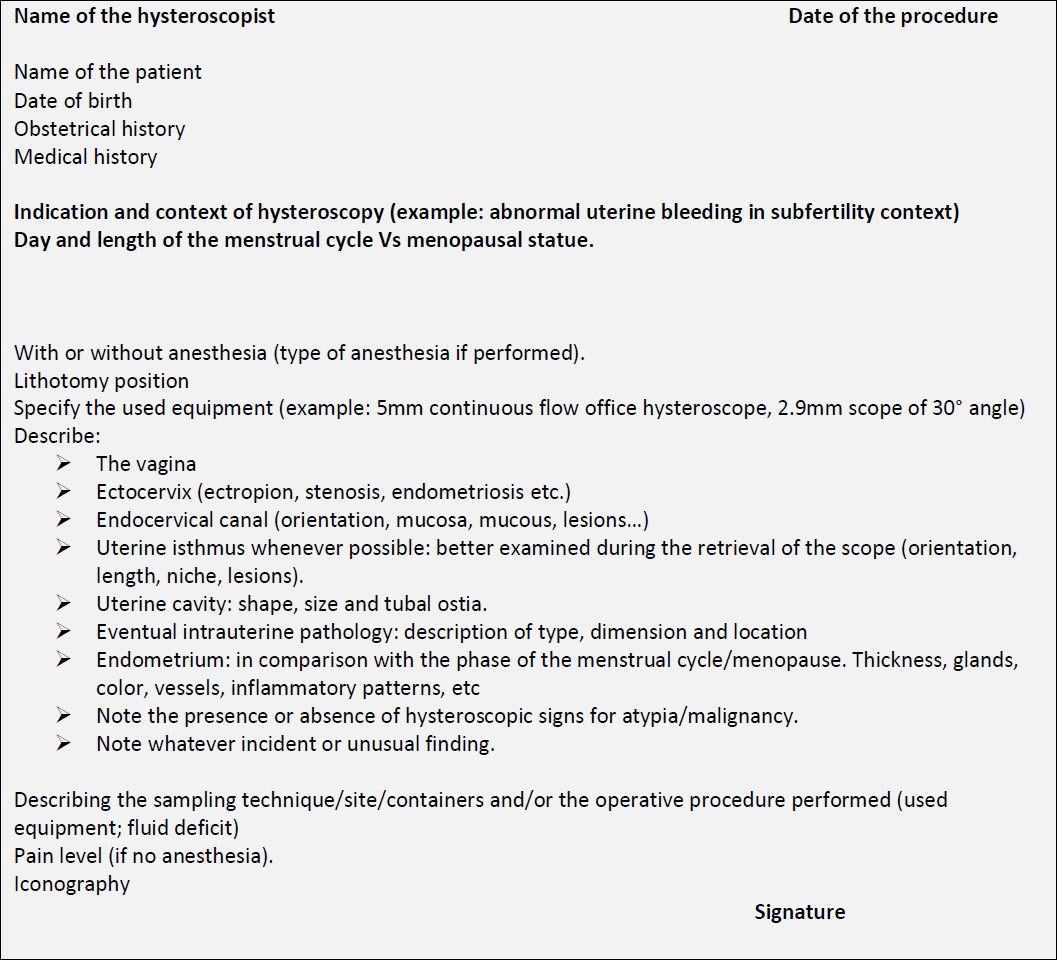
After hysteroscopy, a document reporting all the procedure’s findings with the backing iconography has to be delivered to the patient. In table 2 is proposed a summary descriptive report addressing in a systematic way each step of the procedure.
Conclusion
In the era of “see and treat”, performing a hysteroscopy with a diagnostic sheath just to see and leave has become derisory. An operative sheath with a working channel containing an instrument (graspers or scissors) is the minimum for entry in diagnostic hysteroscopy so that a targeted biopsy and/or removal of pathology are done within the same diagnostic session whenever possible.
The prerequisite for the practice of diagnostic hysteroscopy is growing knowledge of the normal and pathological endometria so as to improve the quality of diagnosis, also by providing good quality visual sampling. The frequently focal character of most benign, premalignant and neoplastic endometrial lesions highlights the importance of a proper sampling within the lesion site, which blind procedures are more likely to miss.
Atlas-like overviews are interesting to visualize in order to memorize the problematic patterns requiring more attention.
References
Figure 1. General features of normal endometrium. A: at histology, with the functionalis (F), the basalis (B) where glands are horizontal and the myometrium (M). B: at hysteroscopy. White arrows: endometrial glands; red arrows: endometrial vessels; stromal cells fill the spaces between glands and vessels.
Table 1: the cyclical changes in proliferative phase (vessels excluded).
Figure 2. . Diagnostic hysteroscopy of normal endometrium at the different phases of the menstrual cycle. A: proliferative phase; 1: early proliferative (thin disorganized, few gland orifices and petechiae); 2: mid proliferative (thicker, pinkish with physiological edema); 3: late proliferative (slightly less thick due to subsided edema, yellowish-pinkish color). B: luteal phase (much thicker, edema, mucosal foldings).
Figure 3. Regular distribution of endometrial glands during proliferative phase.
Figure 4. Uterine isthmus at hysteroscopy. A: nulliparous woman: from the cervix to the cavity; B: post-menopausal woman; C: multiparous woman. Blue: histological internal os of the uterus; green: anatomical internal os of the uterus; Yellow arrow: length of the isthmus.
Figure 5. Endometrial atrophy. A: in post-menopausal women; 1: visible marks of the underlying circular muscular fibers of the myometrium due to advanced atrophy; 2: closer view: scarce glands, cystic in places (c); B: scarce atrophic glands and heterogenous vascularization with Mirena.
Figure 6. Classical acknowledged signs for endometrial inflammation. a: micropolyps; b: strawberry aspect; c: hyperemia; d: stromal edema; e: hemorrhagic spots; f: micropolyps with stromal edema and hyperemia in the fundus.
Figure 7. Additional signs for dysfunctional inflammatory endometrium: irregular distance between gland orifices associated with: a: strawberry aspect; b: edema (on the left) and hyperemia (on the right); c: corrugated irregularly thick area with embossed and debossed patterns; d: a subtle focally thickened mucosa showing typically corrugated surface at increased magnification (embossed and debossed patterns).
Figure 8. Targeted sampling performed within the endometrial areas displaying dysfunctional signs: a: micropolyps and hyperemia; b: strawberry pattern (with the loop); micropolyp+ irregular thickness and gland distribution (with the loop).
Figure 9. Ultrasonographic criteria of adenomyosis by the MUSA group: a: asymmetrical thickening, fan-shaped shadowing; b: cysts, subendometrial buds and lines; c: hyperechoic island, trans-lesional vascularity; d: irregular junctional zone.
Figure 10. Consensually accepted signs for adenomyosis. a: debossed patterns: focal defect in atrophic endometrium; b: focal defect surrounded by strawberry aspect; c:fibrous cystic appearance of intrauterine lesions; d: hypervascularity; e: submucosal hemorrhagic cyst displaying a blue color, releasing a chocolate brown fluid after incision.
Figure 11. Macroscopic classification of polyps: A: pedunculated(1) or sessile (2); B: single (1) or multiple (2); C: various dimensions from millimeters to centimeters; D: various locations: fundus (1), corporeal (2) or isthmic (3). Images by A. Drizi
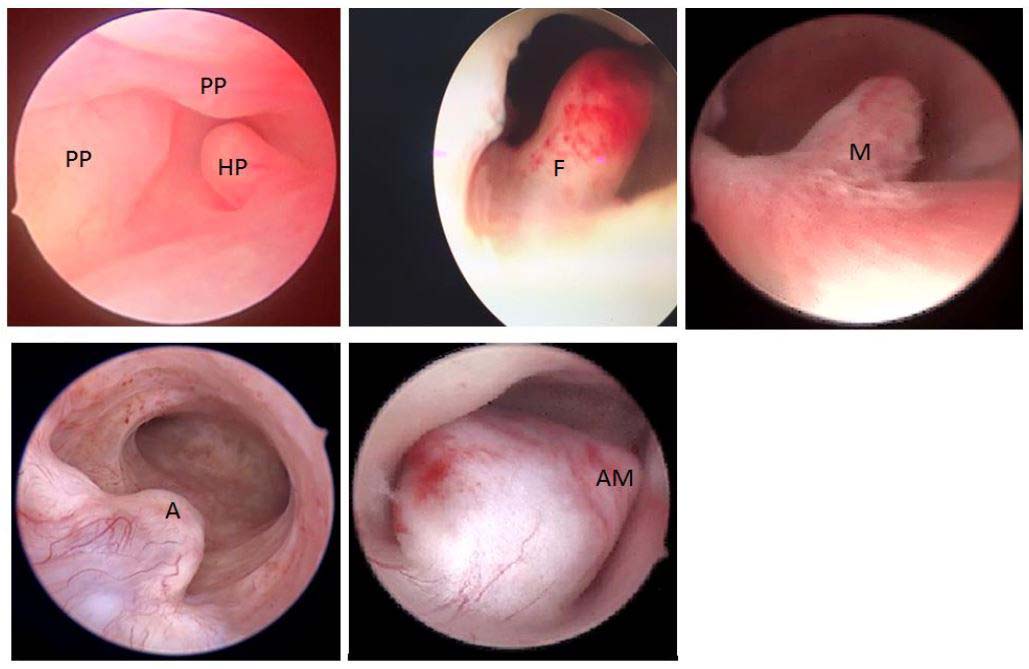
Figure 12. Histopathological classification of polyps. PP: pseudopolyps (temporary in luteal phase); HP: hyperplastic ; F: fibrous ; M: mucosal; A: atrophic (cystic atrophy in a menopausal patient); AM: adenomatous polyp. Images by A. Drizi
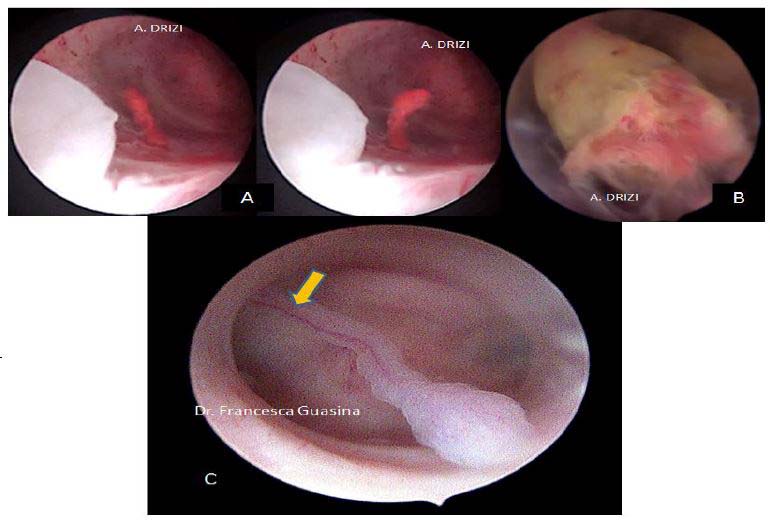
Figure 13. Other types of polyps usually not mentioned in classifications. A: hemorrhagic polyp (massive stromal suffusion of red blood cells); B: necrotic polyp (significant necrosis, yellowish); C: long pedunculated polyp with a unique vascular pedicle seen at hysteroscopy (Courtesy Dr. Francesca Guasina from Italy)
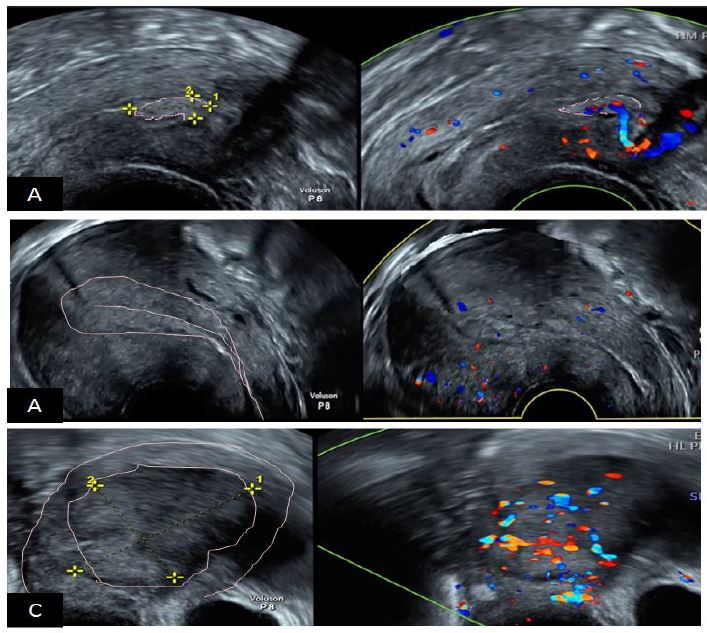
Figure 14. The International Endometrial Tumor Analysis (IETA) consensus opinion regarding the differential diagnosis between EP, EH and EM. A: endometrial polyp hyperechoic with a unique vascular pedicle; B: endometrial hyperplasia with moderate vascularization at Doppler; C: endometrial malignancy, thick image with intense hypervascularization at Doppler. Images by A. Drizi
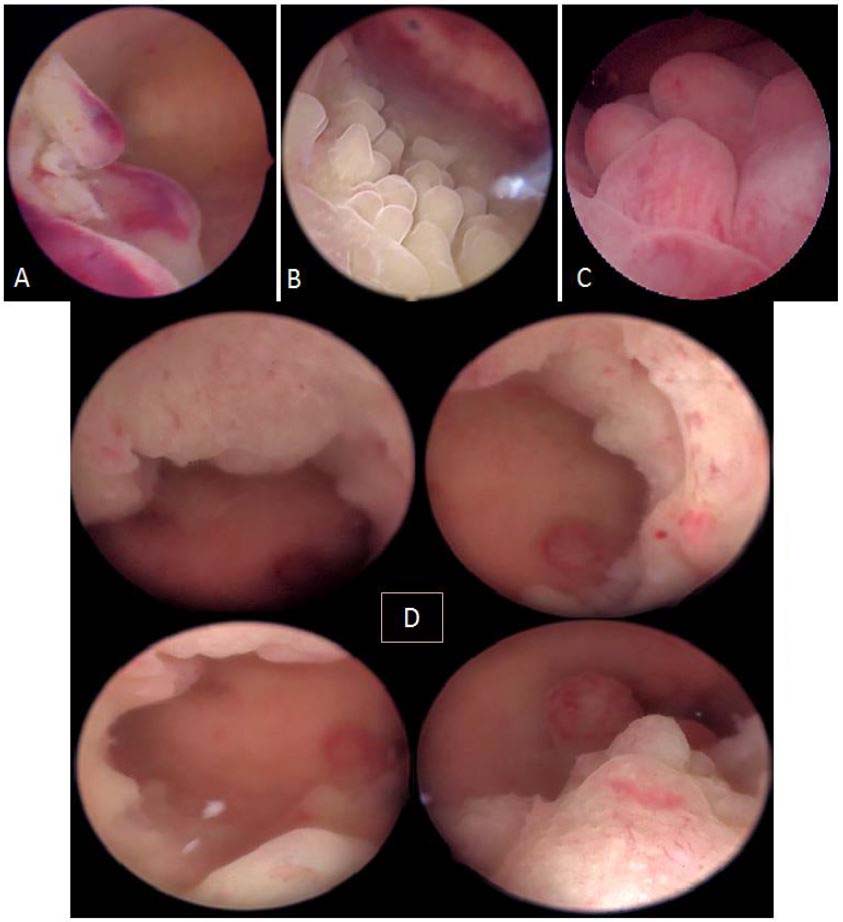
Figure 15. Endometrial hyperplasia without atypia. A: focal; B: papillary; C: polypoid; D: diffuse to the 4 walls of the uterine cavity. Images by A. Drizi.
Figure 16. Uniformly diffuse endometrial hyperplasia without atypia. The major role of ultrasound and of the subtle irregularities of the endometrial surface at hysteroscopy.
Figure 17. Frequently used terminology in classifications based on the morphological appearance of endometrial cancer. A: polypoid (thin pedicles). B: papillary (numerous dendritic projections); C: nodular (bulging); D: glomerular (polypoid with papillary-like component containing atypical neo-vessels covered by a thin layer of endometrial tissue); E: cerebroid (nodular or polypoid with abnormal surface vessels); F: cotton-candy like pattern (necrosis).
Figure 18. The morphological epithelial patterns suggestive of atypical EH/EM requiring attention and sampling. A: irregular surface (polypoid, endophytic or exophytic projections); B: heterogeneous colors: whitish-grayish surface without vessels (w) (back to back glands); yellowish (y) (necrosis); C: yellowish and whitish cotton-candy aspect (necrosis); D: friable hemorrhagic tissue both at hysteroscopic manipulation and at macroscopy.
Figure 19. The morphological vascular patterns suggestive of atypical EH/EM requiring attention and sampling.
A: irregular diameter; B: irregular ramifications and brutal interruptions; C: serpiginous trajectory forming loops at places; D: different atypical vascular patterns at microhysteroscopy. Images by A. Drizi
Figure 20. Specimen processing in histopathology. The main steps.
Table 2: proposed systematic descriptive report.