Authors / metadata
DOI: 10.36205/trocar2.2023004
Abstract
The human endometrium is a unique tissue capable of repeated degradation, loss and self-repair in response to fluctuating levels of sex steroids, in a tightly regulated process. It takes place hundreds of times in a woman’s lifetime, manifesting as vaginal expulsion of blood mixed with cellular debris for a period of several days. The aim of this work is to present a case of en bloc endometrial desquamation in a teenage patient using combined oral contraception. Several mechanisms are proposed, although further studies are needed to better understand this extremely rare phenomenon.
Introduction
Endometrial renewal, as part of the menstrual cycle or following transient withdrawal of exogenous hormones, is a complex and finely tuned process that ensues in response to a decline in serum progesterone and estrogen levels. Under the influence of several cell types, proteolytic enzymes and their mediators, focal tissue degradation and loss precede scar-free regeneration of the outer functional endometrium from basal layer stem cells [1,2]. Desquamation and repair is thought to occur in a discontinuous manner, with areas of shed endometrium coexisting with intact tissue as well as completely repaired epithelium, allowing for a gradual regeneration of the uterine lining over several days [3]. As far as the authors are aware, no cases of en bloc endometrial shedding had been reported until now.
Case report
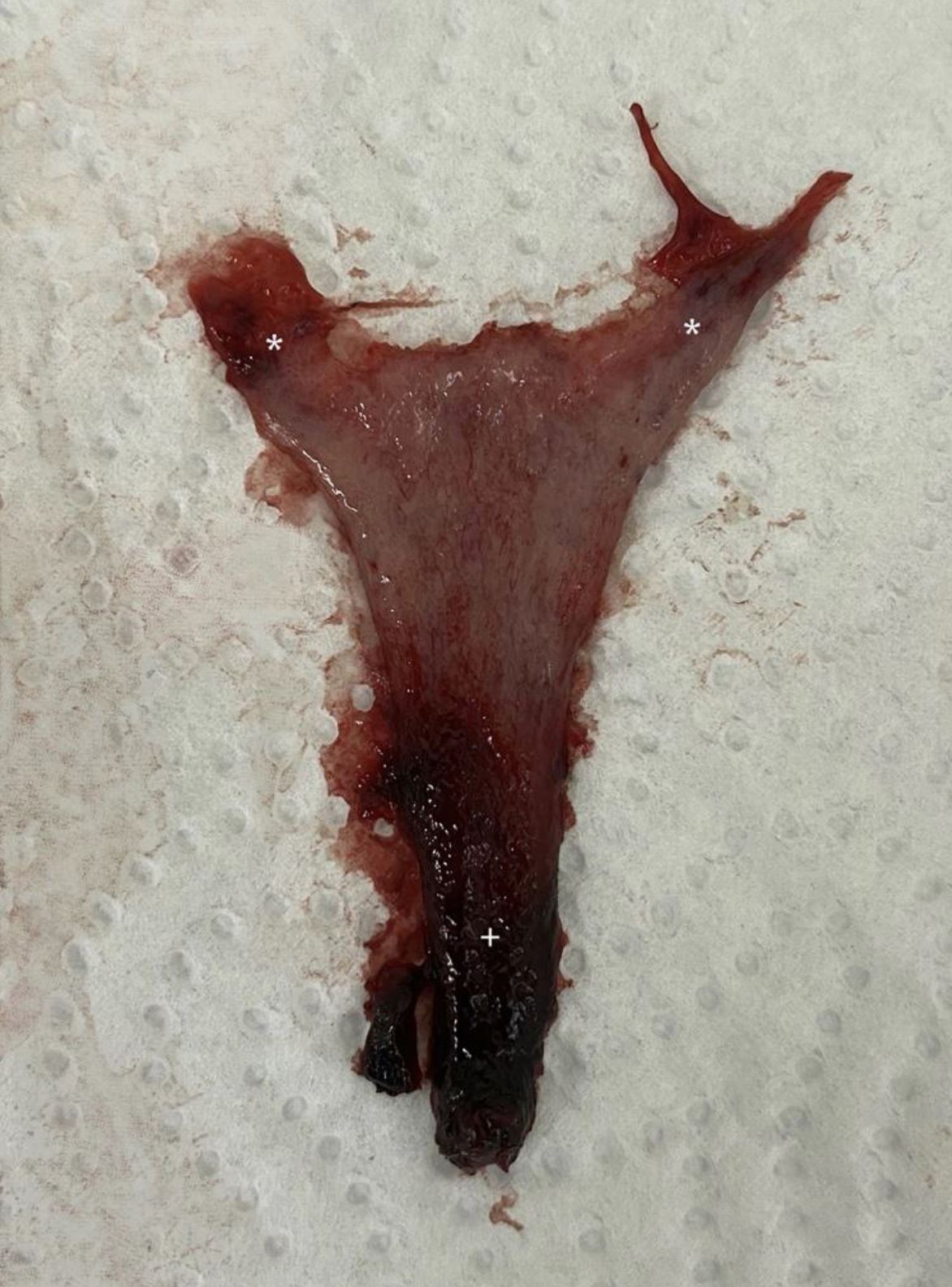
healthy, 18-year-old nulliparous woman anxiously presented to the Emergency Unit after light vaginal bleeding followed by uterine cramping and passage of a pinkish tissue per vagina, which she believed to be an embryo. Despite reporting correct usage of a low dose combined oral contraceptive (in a 21-day regimen) coupled with a barrier method, her monthly withdrawal bleeding was 7 days late. She denied other symptoms or ongoing medication. On examination, the uterus was not enlarged or tender, and there were no palpable masses. Light uterine bleeding was noted on speculum examination. Transvaginal ultrasound revealed a normal uterus with empty cavity and maximal endometrial thickness of 4 mm, as well as normal ovaries with no evidence of pathological adnexal masses. Her serum beta-human chorionic gonadotropin level was <0,6 mUI/mL. On closer inspection, the expelled tissue appeared triangular, similar in size to the uterine cavity, consistent with an entire endometrial leaflet extending from both cornua uteri (*) down to the isthmus (+) [Figure 1]. The pathology report described it as regular decidualized secretory endometrium with interstitial haemorrhage, without gestational components or Arias-Stella reaction. The patient resumed regular withdrawal bleeding the following month, with no recurrences since.
Discussion
The mechanisms and implications of en bloc endometrial shedding are unclear. It is well established that menstruation is the shedding of cellular debris and uterine bleeding that occurs following the degradation and breakdown of the functional layer of the endometrium in response to the decline in progesterone and oestrogen at the end of the menstrual cycle, or a result of withdrawal of exogenous hormones in combined oral contraceptive users [1].
Immediately before menstruation occurs, the endometrium is composed of two layers: an outer functionalis layer — formed by a superficial, compact columnar epithelium (stratum compactum) overlying a multicellular stroma (stratum spongiosum) composed of loose connective tissue, fibroblasts, leukocytes, glands, and spiral arterioles — and a deeper basalis layer, containing networks of glands, vessels and epithelial and mesenchymal stem-cells needed for endometrial regeneration and repair [2,4]. When serum progesterone levels drop, upregulation of NF-𝞳B transcription factor in stromal cells leads to the release of proinflammatory cytokines and chemokines and the production of matrix metalloproteinases (MMPs) by recruited leukocytes and stromal cells [4]. The MMPs have a critical role in the menstruation process by degrading cellular membranes and other components of the extracellular matrix, and their actions are kept in check by the presence of TIMPs (inhibitors of metalloproteinases) [5]. In addition, lysosomal enzymes released by stromal cells also contribute to the damage of cellular membranes and intercellular cadherins [6]. The MMP deregulation or impaired function of lysosomal enzymes could hypothetically lead to an abnormal destruction of the extracellular matrix with an intact stratum compactum, resulting in the pattern of delayed, en bloc shedding that occurred in our patient.
Moreover, progressive breakdown of the functionalis layer is usually accompanied by disruption of the arteriolar vascular system at the natural cleavage plane between the basal layer and the stratum spongiosum, leading to subepithelial interstitial haemorrhage and eventual collapse and shedding of the stroma. This process has been shown to happen in a piecemeal manner across the endometrial cavity, with completely denuded areas coexisting with unshed endometrium as well as newly repaired surface epithelium, as described by Garry et al. in an enlightening hysteroscopic study of the stages of menstruation [3]. This staged shedding appears to confer a survival advantage by limiting the extent of exposed endometrium at any one time, and consequently minimizing the risk of infection and preventing excessive blood loss. One proposed mechanism for this focal endometrial degradation is attributed to endometrial mast cells. These cells, which are spread throughout the functionalis layer, seem to upregulate MMP production by stromal cells and transform precursor MMPs into their active form through the action of tryptase [1], providing a local microenvironment propitious to focal shedding. Sudden and widespread interstitial bleeding at the cleavage plane between the endometrial basal layer and a practically intact functionalis layer (before significant degradation of its extracellular matrix has taken place) could potentially result in the entire shedding of the posterior or anterior endometrial surface. Another possible mechanism for this could reside in the absence or inactivity of endometrial mast cells, precluding focal degradation of the endometrium. Interestingly, the only regions of the endometrial cavity which never shed during menses are the isthmus and the areas around the tubal ostia, which appear to contribute to endometrial reepithelization [7]. These were also the limits of the endometrial tissue presented in our case, as marked by the “*” and “+” symbols. Finally, altered endometrial haemostasis has been associated with abnormal placentation, which is strongly linked to first trimester bleeding, abruption, and preterm birth [8]. It is unclear if our patient’s isolated event would incur an increased risk of the above obstetrical complications in a future pregnancy.
Conclusion
Further clinical and scientific experience are needed to shed light on the rare phenomenon of en bloc endometrial shedding, in order to clarify the possible mechanism behind it and the implications, if any, for these patients’ reproductive health.