Authors / metadata
DOI: 10.36205/trocar5.20240007
Abstract
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and International Deep Endometriosis Analysis group (IDEA) group, the European Endometriosis League (EEL), the European Society of Gynaecological Endoscopy (ESGE), the European Society of Human Reproduction and Embryology (ESHRE), the International Society for Gynaecological Endoscopy (ISGE), the American Association for Gynecologic Laparoscopists (AAGL) and the European Society of Urogenital Radiology (ESUR) elected an international, multi-disciplinary panel of gynaecological surgeons, sonographers and radiologists, including a steering committee, which searched the literature for relevant articles to review the literature and provide evidence-based and clinically relevant statements on the use of imaging techniques for non-invasive diagnosis and classification of pelvic deep endometriosis (DE). Preliminary statements were drafted based on the review of the relevant literature. Following 2 rounds of revisions orchestrated by chairs of participating societies, a first round of voting was carried out. Statements were revised when consensus among societies was not obtained. A second round of voting was organized to evaluate the revised version of the statements.
Twenty statements were drafted out of which 14 reached strong and 3 moderate agreements after the first voting round. The remaining three statements were discussed by all members of the steering committee and chairs of respective societies and rephrased followed by an additional round of voting. At the conclusion of the process, 14 statements received strong and 5 statements moderate agreement with 1 statement left in equipoise. This consensus work aims to guide clinicians involved in treating women with suspected endometriosis during patient assessment, counselling and planning surgical treatment strategies.
This paper has been simultaneously co-published in Ultrasound in Obstetrics & Gynecology, Facts, Views and Vision in ObGyn, The Trocar, Human Reproduction Open, Journal of Minimally Invasive Gynecology and European Journal of Radiology, by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), the European Society for Gynaecological Endoscopy (ESGE), the European Endometriosis League (EEL), the European Society of Human Reproduction and Embryology (ESHRE), the International Society for Gynecologic Endoscopy (ISGE), the American Association of Gynecologic Laparoscopists (AAGL), the International Deep Endometriosis Analysis (IDEA) group, the European Society of Urogenital Radiology (ESUR) and Elsevier B.V.

Introduction
Reducing the diagnostic delay of endometriosis to facilitate timely action requires a shift from a surgically or lesion-oriented diagnosis to a more inclusive diagnosis where – next to symptoms and signs – non-invasive findings at examination and imaging are becoming the main drivers of clinical diagnosis and earlier intervention [1]. Various non-invasive imaging techniques have been advocated over the past decades for non- surgical visualization of pelvic endometriosis. Amongst these, ultrasound (US), primarily in its transvaginal variant, is the most commonly used imaging modality for investigation of women with suspected endometriosis besides magnetic resonance imaging (MRI) and – less commonly – computed tomography (CT) [2]or other radiological techniques suchas barium enema and intravenousurography [3].
The accurate diagnosis of endometriosis with imaging tools, especially in deep endometriosis (DE), which can be observed in approximately 20% of endometriosis cases [4], is of pivotal importance for patient counselling and planning of treatment strategies. Prior to surgery, the diagnosis of DE can be used to predict operative difficulty and, equally important, in the context of infertility, particularly with ovarian endometriosis, it can assist with the guidance of treatment with surgery versus assisted reproductive technologies (ART). The latter is of specific significance with the use of predictive tools, such as the Endometriosis Fertility Index (EFI) [5-8]. Within this, Goncalves, et al. [9] published a study concluding that systematic evaluation of endometriosis by transvaginal ultrasound (TVS) can accurately replace diagnostic laparoscopy, mainly for deep and ovarian endometriosis. This view is also supported by the recently published updated version of the ESHRE (European Society of Human Reproduction and Embryology) Endometriosis Guideline [5]stating that the dogma of the need ofa histological confirmation for diagnosisof endometriosis calls for an urgent needfor a refinement due to the “…advancesin the quality and availability ofimaging modalities for at least someforms of endometriosis on the one handand the operative risk, limited access tohighly qualified surgeons and financialimplications on the other.“
Ideally, patients with severe DE shouldbe referred to tertiary referral centers asthey may benefit from a multidisciplinaryteam consisting of gynecologists,urologists, colorectal surgeons andspecialists in reproductive medicine and imaging [10]. Consequently, the detailed presurgical characterization and classification of endometriosis, especially DE, is of particular importance [4]. Several attempts have been made to evaluate the use of current classification and scoring systems with non- invasive imaging techniques in order to facilitate these processes [11]. Additionally, the environmental impact of non-invasive imaging techniques for endometriosis should also be recognized in times of climate crisis. A recent study by McAllister, et al. [12], calculated the carbon footprint of imaging by MRI, CT and US in Australia.
Comparing the three different modalities, MRI exhibited the largest carbon footprint, followed by CT and US. The impact is mainly attributable to energy consumption and for a smaller part due to consumables. Hence, it should be mentioned that US has the least environmental impact and physicians should be aware when choosing an imaging technique for patients with suspected endometriosis.
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and the International Deep Endometriosis Analysis (IDEA) group, the European Society for Gynaecological Endoscopy (ESGE), the European Endometriosis League (EEL), the International Society for Gynecologic Endoscopy (ISGE), the European Society of Human Reproduction and Embryology (ESHRE), the European Society of Urogenital Radiology (ESUR) and the American Association for Gynecologic Laparoscopists (AAGL) have therefore formed a working group to develop evidence-based statements to guide the use of non-invasive imaging techniques for non-invasive diagnosis and classification of endometriosis in this joint consensus statement. In the present paper, the authors focus on DE. Adenomyosis, ovarian endometrioma, superficial and extra- pelvic endometriosis, adhesions, biomarkers, economic analysis of these techniques and pathohistological and/or surgical methods for classification and diagnosis of endometriosis will not be included in this consensus statement.
Responsibilities
The following statements derive from a consensus process of all listed authors and representatives from the respective societies and do reflect current evidence-based practice and approaches for he non-invasive diagnosis and non-invasive classification of endometriosis using imaging techniques. Clinicians using these statements in everyday clinical practice are strongly recommended to apply independent medical judgement and consider the individual situation and needs of the patient when consulting these statements. All authors listed on this work disclaim any responsibility for their use, application and clinical decisions deriving from the use of these statements.
Methods
The present consensus statement was developed in accordance with a protocol used in a previously published consensus statement [13] involving societies also represented in this work. Using an eight-step protocol chaired and organized by Professors George Condous (G.C.) and Gernot Hudelist (G.H.), an international and multidisciplinary group was established and orchestrated by chairs of respective societies, so-called society working group chairs (G. Condous, ISUOG, IDEA; J. Keckstein, E. Saridogan, ESGE; H. Krentel, G. Hudelist, EEL; C. Becker, C: Tomassetti, ESHRE; B.J. van Herendael, ISGE; M.S. Abrao, M. Malzoni, AAGL; I. Thomassin-Naggara, ESUR) all together involving 53 experts with extensive expertise in the field of diagnosis and/or surgical treatment of endometriosis reflected by research, clinical expertise and administrative responsibilities and society leadership positions. The list of authors finally consisted of 10 radiologists with a special interest and expertise in MRI and TVS, 12 gynecologists with a special interest and expertise in gynecological ultrasound, 13 gynecologists with extensive experience in surgery for DE and gynecological ultrasound and 18 gynecologists exclusively focusing on surgery for DE.
A systematic literature review of relevant studies published from inception to February 2023 was carried out by the coordinating chairs (G.C., G.H.) and the joint first author Bassem Gerges (B.G.) using the MEDLINE, Embase, Google Scholar, PubMed and Scopus databases (Appendix 1). The literature search was limited to publications in the English language. Editorials, letters and case reports were excluded, priority was given to systematic reviews, meta-analyses and validating cohort studies. The reference list of each identified article was additionally reviewed for other potentially relevant articles. The main chairs (G.C, G.H.) and joint first author (B.G.) formulated the preliminary consensus statements and were responsible for the first draft of this work. This was followed by distribution to respective society chairs who again distributed and discussed the preliminary consensus statement with all group members followed by a first round of revisions coordinated by the representatives of each society. Statements were modified in cases of lacking consensus among group members. The respective group members had the opportunity to provide comments/suggestions with their resubmitted versions of the draft. The society working group chairs then submitted the results and comments of the first draft to the main coordinating chairs (G.C., G.H.) and joint first author (B.G.) and suggested revisions of the statements if necessary. The revised version of the statement was resubmitted to working group chairs and thereby all group members and the process was repeated. Based on the results of the second round, the work and respective consensus statements were finalized resulting in 20 statements achieved during this process. Society group members were then able to vote binary (agree/disagree) and abstain from voting in cases of conflict of interest. Society group members were then able to vote binary (agree/disagree) and abstain from voting in cases of conflict of interest. Statements were classified as strong agreement (more than 80% agree), moderate agreement (more than 60% agree), equipoise (40%-60% agree), or disagreement (less than 40% agree). A very final version of the document was then resubmitted to all group chairs of respective societies for final approval. The summary of the supporting evidence (Appendix 2), all final consensus statements and their levels of evidence and grades are presented in this work.
Results
Transvaginal sonography (TVS)
Rectosigmoid DE
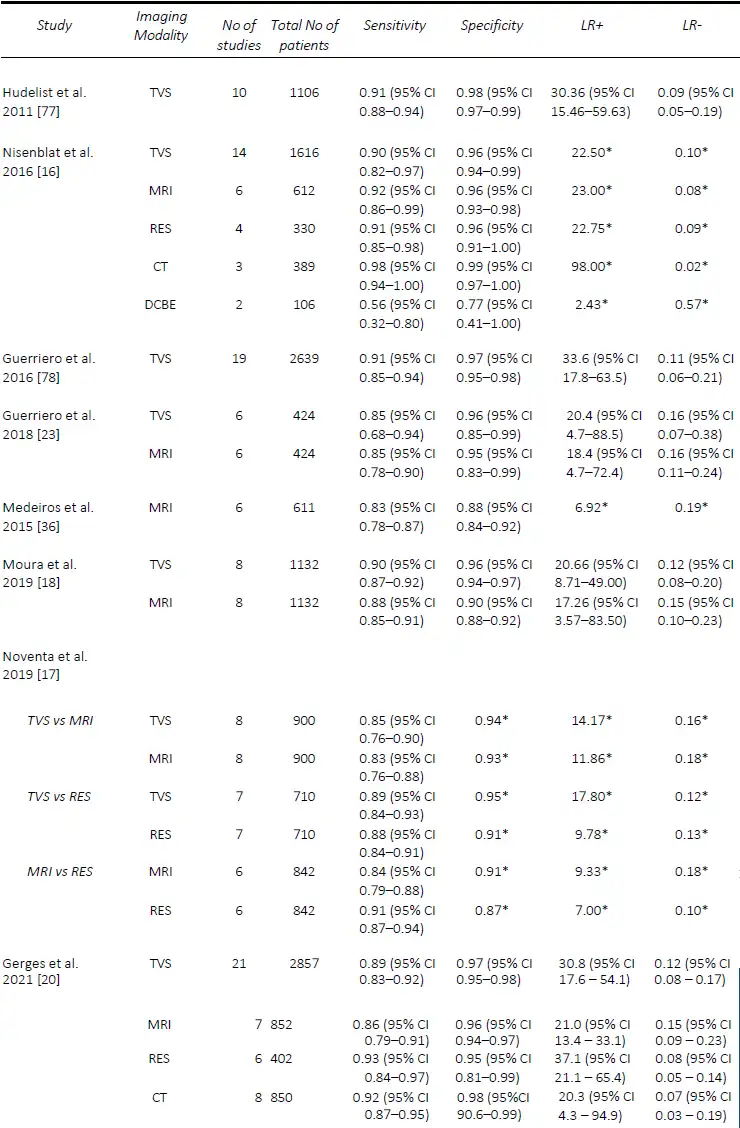
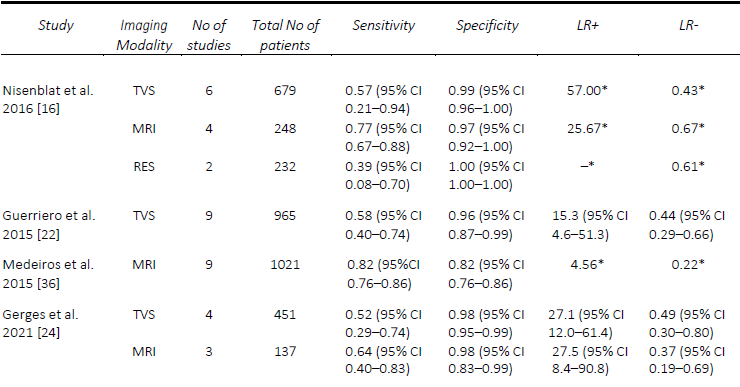
Since Bazot, et al. [14] correlated the ultrasound and surgical findings of deep pelvic endometriosis, there has been a considerable number of studies published pre- operatively assessing imaging techniques for the presence of DE, in particular rectosigmoid DE. Of these, TVS is the most studied, often used as the first-line modality given its accessibility, relatively low cost and non-invasiveness [15]. In the Cochrane review published in 2016 by Nisenblat, et al. [16], which included 14 studies, the overall pooled sensitivity and specificity for TVS was 90% and 95% respectively. In 2019, Noventa, et al. [17] performed a meta-analysis of only head-to-head TVS versus MRI studies and found the sensitivity of TVS to be 85%. Subsequently, there were two well-conducted meta-analyses, although they included a small number of studies, specifically 8 [18] and 11 [19]. Moura, et al. [18] performed a meta-analysis comparing TVS and MRI for the diagnosis of rectosigmoid DE in the same population, both of which had sensitivities and specificities of 90% and 96%, respectively. In 2020, Pereira, et al. [19]published a comparative study ofTVS and MRI, including comparisons ofenhanced techniques, and reportedsensitivities and specificities of 80% and94% for the former. Most recently, in2021, Gerges, et al. [20] performed asystematic review and meta-analysis ofprospective studies limited to those withat least 10 affected/unaffected patientsand found an overall pooled sensitivity ofall studies assessing TVS (21 studies) of 89%, and specificity of 97%. Furthermore, in their sub-group analysis of 2-D TVS (13 studies) and TVS with rectal water contrast (5 studies), the sensitivities and specificities were comparable at 84% and 97% versus 88% and 97%, respectively. A comparison of the included meta-analyses for the detection of rectosigmoid DE is summarized in Table 1.
Uterosacral Ligament/Torus uterine (USL), Rectovaginal Septum (RVS) and Vaginal DE
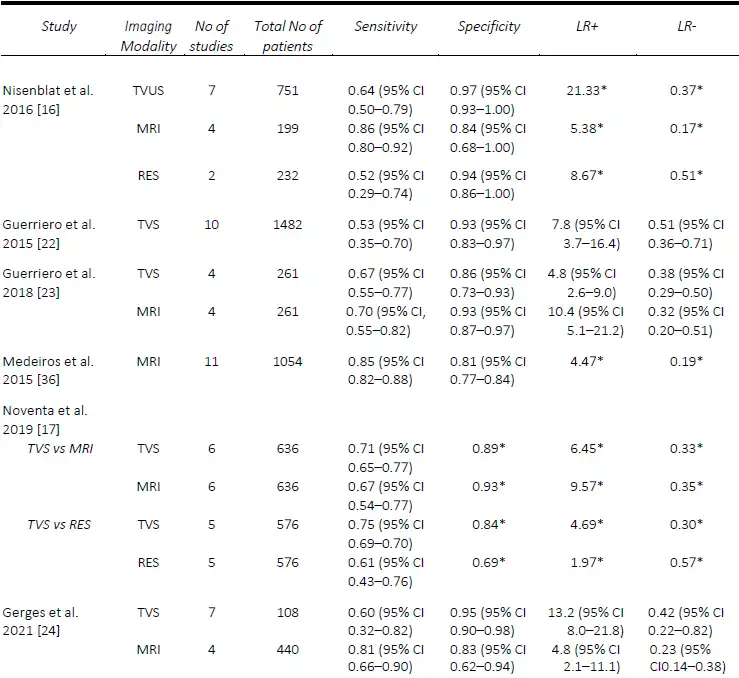
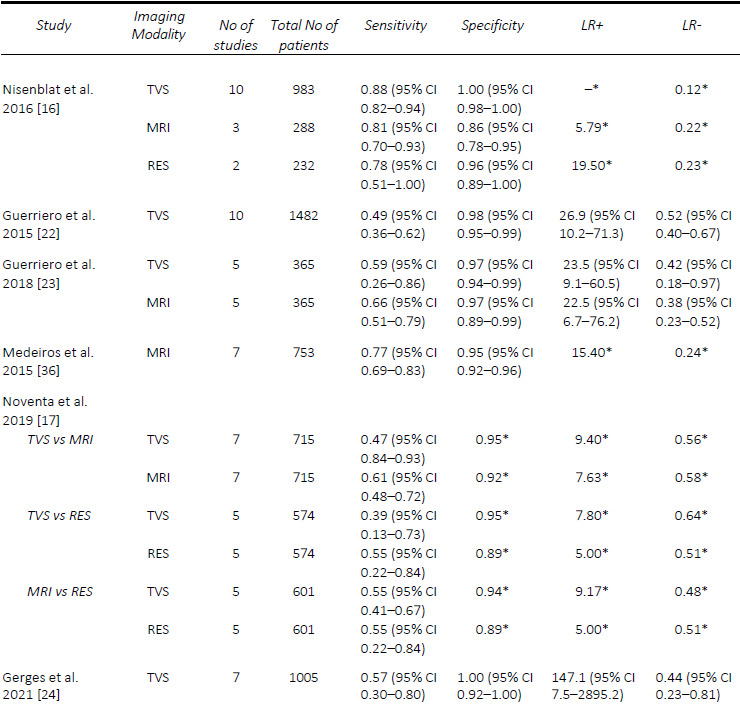
AAssessment of USL DE via TVS seems to be one of the most challenging, despite DE in this region being one of the most common sites, found in up to 61% of patients at laparoscopy [21]. The performance of TVS for the pre-operative diagnosis of USL DE is relatively comparable in published meta-analyses. The first of these, in 2016, by Nisenblat, et al. [16] compared all imaging modalities and obtained a sensitivity and specificity of 64% and 97%, respectively, from a total of seven studies. Guerriero, et al published two reviews, the first in 2015 which assessed TVS, and included 11 studies, found a sensitivity and specificity of 53% and 93% [22], whilst in the more head-to-head recent review published in 2018, of which six studies were included, the sensitivity and specificity was 67% and 86%, respectively [23]. These results were slightly lower than the head-to-head review by Noventa, et al. (13) in 2019, from which the sensitivity of TVS was 71%, likely due to the inclusion of retrospective studies. The most recent systematic review and meta-analysis in 2021 by Gerges, et al. [24], which included all prospective studies assessing all imaging modalities, found pooled sensitivities and specificities of 60% and 95%.
Similarly, the performance of TVS for the detection of RVS and vaginal DE was poorer, particularly when compared to MRI. In the first review by Guerriero, et al. [22], the sensitivity and specificity of TVS for RVS DE was 49% and 98% and vaginal DE was 58% and 96%, respectively. The results were quite similar for RVS DE in the two head- to-head reviews, with Guerriero, et al. [23] finding a sensitivity and specificity of 59% and 97%, and Noventa, et al. [17] reporting a sensitivity of 47% and a specificity of 95%. Most recently, Gerges, et al. [24], reported overall pooled sensitivities and specificities of 57% and 100% for RVS DE (7 studies) and 52% and 98% for vaginal DE (four studies), respectively. A comparison of the included meta-analyses for the detection of USL, RVS and vaginal DE are summarized in Tables 2-4. Since the IDEA consensus opinion in 2016 [25, 26], there has been further delineation of the anatomical terminology used in diagnostic imaging to define the parametrium, paracervix and uterosacral ligaments [27-29]. This is of particular significance as parametrial DE can be associated with ureteral stenosis, with associated increased operative risks and the potential need for multidisciplinary surgery. In 2021, Guerriero, et al. [30] published a systematic review and meta-analysis of the accuracy of TVS for the detection of parametrial DE, which included four studies. The pooled sensitivity was 31% and the specificity was 98%, although a positive result on TVS significantly increased the post-test probability from 18% to 79%. More recently, in a retrospective review, Roditis, et al [31], found the sensitivity and specificity for the detection of parametrial DE to be 20.7% and 97.1% for TVS, and 36% and 93.8% for MRI.
Bladder DE
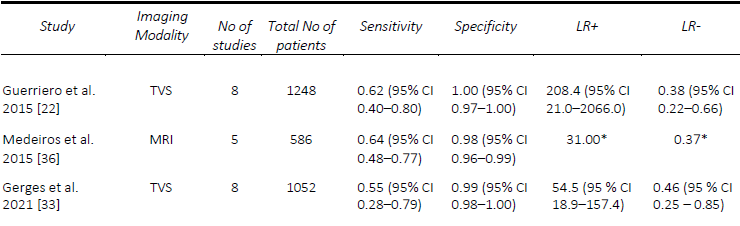
DE involving the urinary tract, namely the bladder, ureters and kidneys, is a form of DE affecting between 19-53% of women with pelvic DE, but only 1-2% of people affected by endometriosis [32]. Given the low incidence of this specific manifestation of DE, there are limited systematic reviews assessing the pre-operative diagnostic accuracy of imaging specific to the bladder DE. In 2015, Guerriero, et al. [22] performed a systematic review including prospective and retrospective studies with at least 50 participants who underwent TVS prior to surgery and found a pooled sensitivity and specificity were 62% and 100%, respectively. In 2019, Noventa, et al. [17]performed a systematic review onhead-to-head studies, including retrospective studies, with only two studies that compared TVS and transrectal endoscopic sonography (RES). They found, by univariate analysis, diagnostic odds ratios of 4.94 for TVS and 3.13 for RES. In a review of prospective studies of all imaging modalities, with at least ten affected and unaffected patients, Gerges, et al. [33] found an overall pooled sensitivity of 55%, specificity of 99%, although a meta-analysis was not able to be performed given the limited number of applicable studies. A comparison of the included meta-analyses for the detection of bladder DE is summarized in Table 5.
Magnetic resonance imaging (MRI)
Rectosigmoid DE
With regards to rectosigmoid DE, in 2016, Nisenblat, et al. [16] included a total of six studies with an overall sensitivity and specificity of 92% and 96%. More recently, in 2019 Noventa, et al. [17] performed a meta-analysis of only head-to-head studies and found the pooled sensitivity and specificity for MRI of 83% and 93% when compared to that of TVS at 85% and 94%, and 84% and 91% when compared to RES at 91% and 87%. Moura, et al. [18] performed a meta-analysis comparing MRI and TVS in the diagnosis of rectosigmoid DE in the same population. Both modalities were found to have similar sensitivity and specificity of 88% and 90%, and 90% and 96%, respectively. In 2020, Pereira, et al. [19]published a comparative study ofMRI and TVS, including comparisons of enhanced techniques, and reported sensitivities and specificities of 82% / 94%, and 80% / 94%, respectively. However, the latter two meta- analyses [18][19], although well conducted,included a small number of studies, namely eight and eleven, respectively. More recently, in 2021, Gerges, et al. [20] performed a systematic review and meta-analysis of prospective studies limiting studies to those with at least 10 affected/unaffected patients found the sensitivity and specificity of all studies assessing MRI (7 studies; 852 patients) to be 86% and 96%, whilst the sub-analysis of 2D MRI (5 studies; 813 patients) was very similar with a sensitivity and specificity of 85% and 96%. Due to the limited number of studies, sub- analyses were not performed. In a study assessing interobserver agreement, 3-D MRI performed similarly to 2-D MRI for the detection of rectosigmoid DE, with sensitivities and specificities between radiologists ranging from 89-100% and 94-100%, [34], while MRI with rectalultrasound gel outperformed 2-D MRI with a sensitivity of 99% and specificity of 96% [35]. A comparison of the included meta-analyses for the detection of rectosigmoid DE is summarized in Table 1.
Uterosacral Ligament/Torus uterinus (USL), Rectovaginal Septum (RVS) and Vaginal DE
MRI generally outperforms TVS for the detection of USL DE. Nisenblat, et al. [16] compared all imaging modalities and found sensitivities and specificities of MRI (4 studies) for the detection of USL DE of 86% and 84%, compared with 64% and 97%, respectively, for TVS (7 studies). In the head-to-head review in 2018 by Guerriero, et al. [23], a total of six studies were included, from which the sensitivity and specificity, respectively, for the detection of USL DE for MRI was 70% / 93% compared with 67% / 86% for TVS. Similarly, with RVS DE, the sensitivity and specificity for MRI was 66% and 97% compared with 59% and 97% for TVS. In contrast, Noventa, et al. [17] performed a head-to-head meta-analysis including retrospective studies and found TVS to be slightly superior to MRI with sensitivities and specificities of 71% / 89% and 67% /93%, for the detection of USL DE. In contrast, the sensitivities and specificities for the detection of RVS DE were 47% / 95% for TVS and 61% / 92% for MRI. In a meta-analysis assessing the performance of MRI, Medeiros, et al. [36] reported sensitivities and specificities for USL DE, RVS DE and vaginal DE of 85% / 80%, 77% / 95% and 82% / 82%, respectively. Similarly, the meta-analysis of prospective studies by Gerges, et al. [24] found MRI to consistently outperform TVS with sensitivities and specificities for USL DE of 81% / 83% and 60% / 95% respectively, for vaginal DE of 64% / 98% and 52% / 97%, respectively. A comparison of the included meta-analyses for the detection of USL, RVS and vaginal DE are summarized in Tables 2-4.
Bladder DE
The studies assessing the diagnostic accuracy of imaging techniques for bladder DE are quite limited, largely due to the low incidence of the disease. Medeiros, et al. [36] reviewed MRI for the diagnosis of bladder DE including both, retrospective and prospective studies allowing them to perform a pooled analysis for the detection of bladder DE. They found a pooled sensitivity and specificity of 64% and 98%, respectively. In a review of prospective studies [33], while pooled analyses could not be performed due to the limited number of studies, there were two which assessed 2- D MRI with reported sensitivities ranging from to 50% [37] to 100% [38] and specificities ranging from to 97% [37] to 100% [38]. Within this, MRI with rectal ultrasound gel performed similarly with a sensitivity of 70% and specificity of 100% [35]. A comparison of the included meta-analyses for the detection of bladder DE is summarized in Table 5.
Computed tomography (CT)
The use of CT for the pre-operative detection of endometriosis is less studied than TVS and MRI, mostly used for the detection of rectosigmoid DE. In the 2021 systematic review by Gerges, et al [20], six studies were included which assessed standard CT (402 patients), with three assessing CT [39-41] and three assessing CT with water enema [42-44]. The overall pooled sensitivity and specificity of CT for the detection of rectosigmoid DE were 93% and 95%. Sub-analyses of CT colonography were not performed, although the results ranged widely with one study [42] performing poorly with a sensitivity and specificity of 68% and 67%, compared with the other two publications, ranging from 93 – 95% and 87 – 93% [43, 44]. In the review by Nisenblat, et al in 2016 [16], these results were improved when CT was combined with water enema, with three studies (389 patients) [40-42] included, resulting in a pooled sensitivity and specificity of 98% and 99%, respectively. However, the authors did state that this technique should be avoided in young patients whenever possible due to the radiation exposure [45]. This is consistent with the “ALARA” principle of ensuring that the exposure to radiation is “as low as reasonably achievable” [46].
General remarks on imaging
The test performance of any imaging technique is operator dependent and will increase with exposure, level of training and skills and experience of the operator. Also, as systematic reviews, per definition, include older studies, and because the expertise in endometriosis imaging of endometriosis has dramatically improved worldwide in the last few years, it can reasonably be assumed that the published sensitivity figures are an underestimation of the current status. Consequently, the following statements should be interpreted based on these assumptions. Also, whilst these imaging techniques have been compared to each other in the various anatomical areas above, they can be complimentary and do not need to be used exclusively [3]. Within this, a recent analysis of the combined use of vaginal palpation, TVS and MRI with at least two positive tests was observed as the most valid model for diagnosing DE with an accuracy of 91.4% [47].
Non-invasive use of classification
Non-invasive use of classification and scoring systems for endometriosis: (#)Enzian, AAGL score, Endometriosis Fertility Index (EFI), deep Pelvic Endometriosis Index (dPEI), revised American Society of Reproductive
Medicine (rASRM) score, and Ultrasound Based Endometriosis Scoring System (UBESS)
Classification and scoring systems for topographical description and extent of endometriosis and associated secondary adhesions have been proposed and used in multitude over decades with varying rates of recognition amongst clinicians, radiologists, sonographers and gynecological surgeons [48].
TVS for description and classification of DE
Terms and definitions for uniform and standardized description of DE across different centers and countries have been proposed by the IDEA group and have been consequently widely accepted [25]. These definitions primarily serve as a standardized terminology for describing DE with ultrasound. Their use, applicability and accuracy as well as reproducibility is currently under investigation in an international multicentered study level. Within this, Leonardi et al. [49] recently published the results of a pilot study on the accuracy of IDEA terms and definitions for presurgical detection of DE. Two-hundred and seventy-three women were included, out of which 256 (93.8%) had endometriosis with 190 (74.2%) DE cases. In these women, the diagnostic accuracy was 86.1%; sensitivity, 88.4%; specificity, 78.8%; positive predictive value (PPV), 92.9%; negative predictive value (NPV), 68.4%; LR+, 4.17; LR-, 0.15. Within this, Szabo et al. [26] demonstrated a diagnostic accuracy, sensitivity, specificity, NPV, PPV, LR+ and LR- of 94%, 93.5%, 94.6%, 93.1%, 94.9%, 17.24 and 0.07, respectively, for TVS diagnosing colorectal DE applying the IDEA criteria in 537 women with suspected endometriosis.
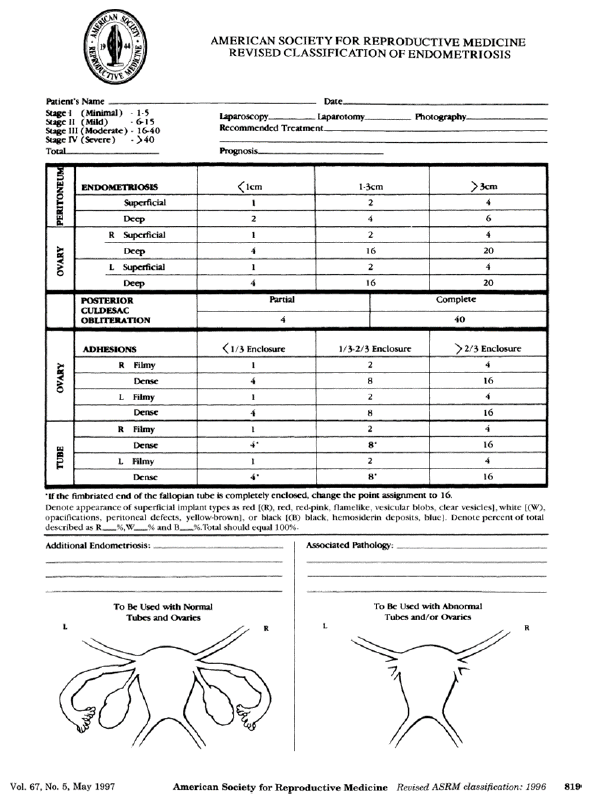
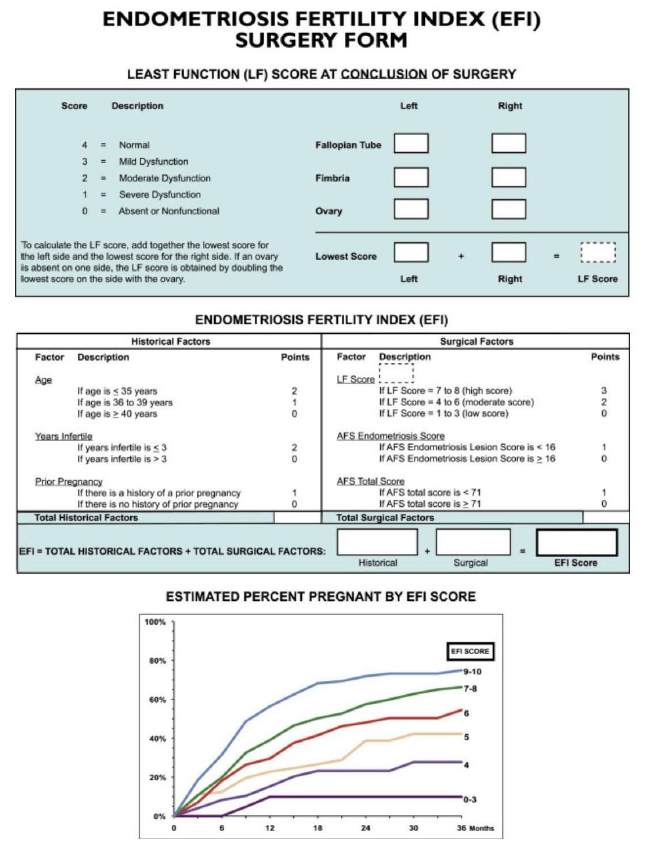
Amongst all scoring and/or classification systems published so far, the revised American Society of Reproductive Medicine (ASRM) score [50] (Figure 1), the (#)Enzian classification [51], [52] (Figure 2), the Ultrasound based Endometriosis Staging System (UBESS) [53] (Figure 3), the Endometriosis Fertility Index (EFI) [6, 8] (Figure 4) for prediction of conception following surgery for endometriosis and the AAGL Endometriosis Classification [54] have also been investigated for their non- invasive applicability using TVS and/or MRI. In the ideal scenario, description of endometriosis via scoring and classification systems should be possible for surgeons and radiologists and/or sonographers to speak one common language to facilitate communication and clinical research.
As a consequence, there have been efforts to investigate the possibility of using the rASRM score with TVS. The score divides grades of severity of endometriosis into 4 stages – minimal, mild, moderate and severe taking into account endometriotic lesions affecting the pelvic peritoneum, ovaries and associated adhesions. Points are counted and added to a score dependent whether the lesion is deep or superficial, the size of the endometriotic lesion, and the type (filmy or dense) and extent of adhesions involving the fallopian tubes, ovaries, and the pouch of Douglas. Leonardi et al. [55] retrospectively investigated the accuracy of TVS for staging of endometriosis pre- operatively in 204 patients using the rASRM classification. When evaluating stages separately, sensitivities, specificities, PPVs and NPVs of TVS were 18.2%, 94.7%, 80% and 49.7% for rASRM stage 1; 22.7%, 96.7%, 45.5% and 91.2% for stage 2; 62.5%, 92.0%, 40.0% and 96.7% for stage 3; and 71.9%, 97.1%, 82.1% and 94.9% for stage 4. Similar to Leonardi et al. who observed lower accuracies for TVS in minimal and mild rASRM stage disease, Holland et al. [56]found a low sensitivity for TVSdiagnosing minimal and mildendometriosis but an accuracy of 94% forTVS for detecting moderate and severedisease. Of note, both authors observedlow diagnostic accuracy for TVS in thedetailed assessment of DE due to the factthat DE could not be clearly scored usingthe rASRM classification. Finally,Tomassetti et al. [6]found good agreement using TVS forestimating the Endometriosis FertilityIndex (EFI) which is partly based on therASRM. So far, there have been noattempts to use MRI in combination with the rASRM score to describe and diagnose endometriosis.
To better describe DE using a classification system, the ENZIAN classification was developed in 2003 [51] and further extended and modified in 2021 [52]. So far, five studies have evaluated the accuracy of TVS in combination with the ENZIAN classification. Hudelist et al. [57] compared TVS findings with surgical findings in 195 women with DE and found good agreement between these modalities especially for ENZIAN compartments A (vagina, rectovaginal space), C (rectum) and FB (urinary bladder. TVS detected DE in compartments A, B, C, and FB with sensitivity 84%, 91%, 92%, and 88%, respectively, and specificity 85%, 73%, 95%, and 99%. Recently, Enzelsberger et al. [58] evaluated the preoperative use of the ENZIAN classification using TVS and/or MRI in a prospective multicenter study including 1062 women undergoing surgery for endometriosis observing lower accuracies for TVS and/or MRI for compartments A, B and C.
An exact concordance regarding compartment and grade 1, 2 or 3 was observed in 369 women (35.14% of 1050 valid ratings) which increased to 40.3% when categorizing the numerical ratings in compartments A/B/C into ‘affected’ (combining values 1, 2 and 3) and ‘not affected’ (0 coded). Overall consistency, sensitivities, specificities, PPVs and NPVs for compartment A were 83%, 63%, 91%, 72% and 88%; compartment B 69%, 47%, 86%, 72%, 68% and C 89%, 52%, 96%, 76% and 91%, respectively. However, it needs to be mentioned that MRI or TVS could be applied and that TVS was also performed by sonographers with limited experience in scanning DE which limit the results of the study regarding the accuracy of TVS when used in combination with the ENZIAN classification.
In order to test the accuracy of the modified, so-called #ENZIAN classification which also takes into account peritoneal and ovarian endometriosis and secondary tubal adhesions and has been shown to outperform the ASRM score regarding the description of DE [59], Di Giovanni et al. [60] retrospectively investigated 93 patients undergoing TVS using the #Enzian classification followed by surgery and observed sensitivities and specificities for TVS – verified endometriosis in compartments O (ovary) right/left: 100% and 100%/100% and 96%, A: 97% and 86, B right/left: 100% and 90%/97% and 70%, C: 100% and 96%, FB: 86% and 100%, FI (intestines): 100% and 100%, and FU (ureter): 100% and 100%, respectively. Similarly, Bindra et al. [61] retrospectively reviewed 50 patients undergoing surgery following TVS mapping used with #Enzian observing similar accuracy values. Recently, Montanari et al. [62] evaluated the use of the #Enzian classification in a prospective, multicentered study including 745 patients undergoing TVS and surgery for DE. The sensitivities for the detection of endometriotic lesions ranged from 50% (#Enzian compartment FI – other intestinal locations) to 95% (#Enzian A), specificities from 86% (#Enzian T left) to 99% (#Enzian FI) and 100% (#Enzian FB – urinary bladder, FU -ureters and FO – other extragenitallocations) with positive predictive valuesof 90% (#Enzian T right) to 100% (#ENZIAN FO), negative predictive values of 74% (#ENZIAN B left) to 99% (#ENZIAN FB and FU) and accuracies of 88% (#ENZIAN B right) to 99% (#ENZIAN FB) underlining that presence and extent of DE can be accurately evaluated using TVS in combination with the #ENZIAN classification.
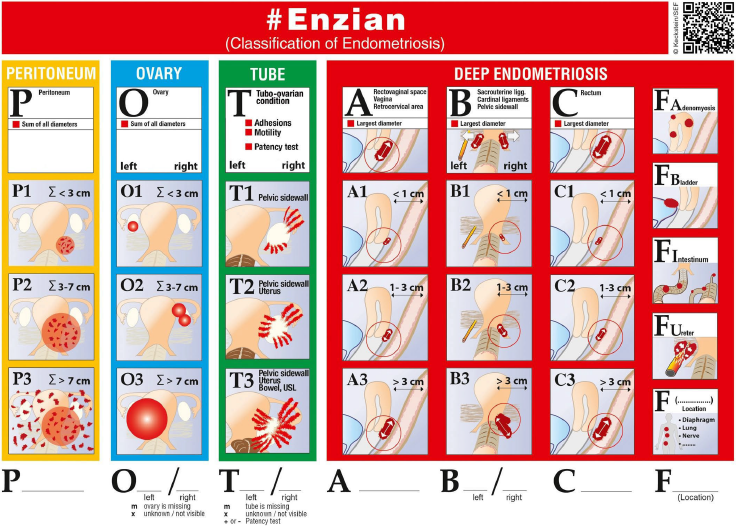
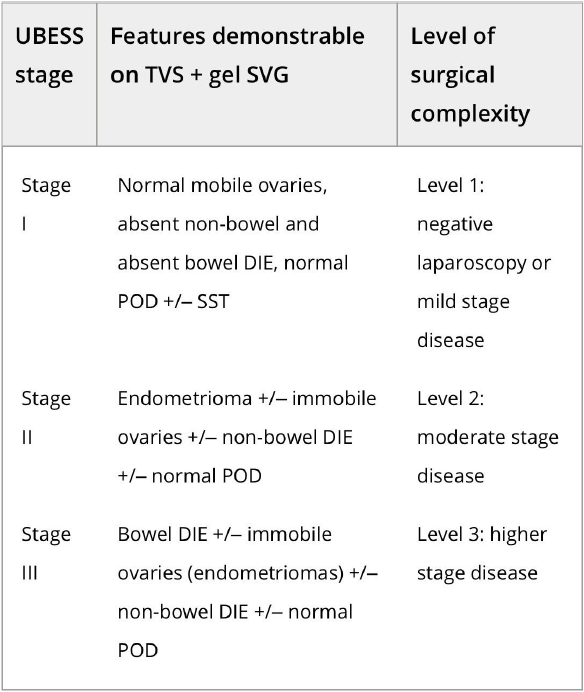
In order to stage disease extent and predict the complexity of surgery in patients with DE, the UBESS was created based on anatomical locations of DE and sonographic markers of local invasiveness [53]. In a multicenter prospective and retrospective cohort study on 192 consecutive women with suspected endometriosis, three stages of UBESS (I-III) were correlated with the three levels of complexity of laparoscopic surgery.
The need (accuracy, sensitivity, specificity, positive and negative predictive values and positive and negative likelihood ratios) for advanced laparoscopic surgery reflected by UBESS stage III were 95.3%, 94.8%, 95.5%, 90.2%, 97.7%, 21.2 and 0.054, respectively [53]. External validation of the UBESS showed little predictive value for surgical difficulty of the UBESS in a small number of 33 patients [63] and problems with generalizability in cases lacking bowel DE or obliteration of the pouch of Douglas [64].
Amongst other classification and scoring systems that have been proposed [48], the recently published AAGL classification [54] and the EFI [8] should be mentioned. Recently, Abrao, et al. [65] evaluated the AAGL Endometriosis Classification by ultrasound and showed that the sonographic estimation of the 2021 AAGL Endometriosis staging is greatest in AAGL stages 1 and 4 and reliably distinguishes AAGL stages 1/2 from 3/4. They found that ultrasound best identified endometriosis of the ovaries, bladder, and bowel but was more limited for the tubes and superficial peritoneum. The EFI primarily works as a model to predict fertility outcomes following surgery for endometriosis. It constitutes of a 10-point scoring system based on factors such as patient characteristics (age, duration of infertility and history of prior pregnancy), the rASRM classification and functionality of fallopian tubes and ovaries during surgery. So far, one study demonstrated the possibility of applying the EFI with ultrasound instead of invasive methods showing that the prediction model can be assessed using TVS-based tubal patency testing with a 10% loss of accuracy compared with the invasive EFI [6].
MRI for description and classification of DE
Two consensus MRI lexicons [66, 67] from the Society of Abdominal Radiology (SAR) and from the French Society of Women’s Imaging (SIFEM) were recently published. In these two MRI consensus lexicons, the different locations of DE are described according to a compartment-based approach of the pelvic. The most recent one emphasized the description of lateral compartments which are usually difficult to detect with TVS and crucial for surgical planning.
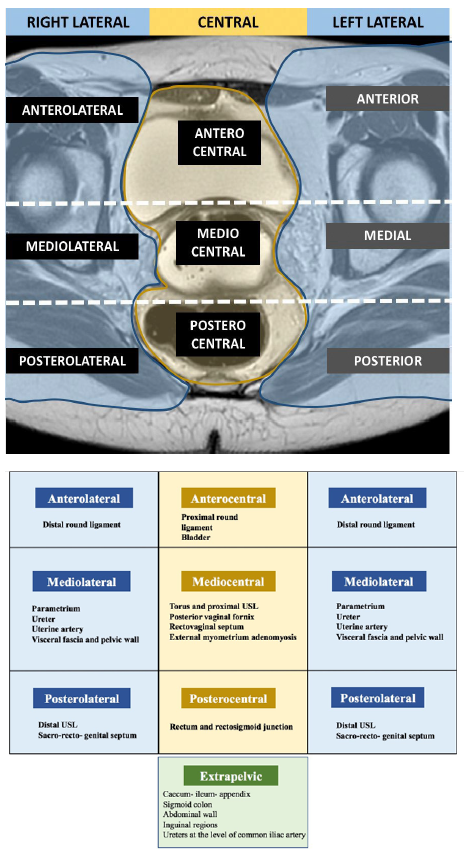
To date, seven studies investigated the use of the ENZIAN classification with MRI with good agreement rates between radiological and surgical findings except for B compartment lesions [68], [69], [70], [71]. Manganaro, et al. [72] and Burla, et al. [73] showed that the ENZIAN classification based on MRI findings is also reproducible. In addition, Thomassin-Naggara, et al. [74] also demonstrated that DE lesions in compartment A and C with ENZIAN classification were accurate in predicting operating time, hospital stay and post operative complications according to Clavien-Dindo. However, Thomassin-Naggara et al. highlighted the poor reproducibility of the description of B lesions due to the difficulty of measuring USL on MRI. The same limitation was described in a recent prospective international multi-center study performed in 12 centers and 1062 women [75]which demonstrated that the MRIbased and surgical ENZIANclassifications were concordant for DElesions in compartment A in 78.7%(118/150), for C lesions in 82.7%(124/150) but only in 34.7% (52/150) forB lesions. In this setting, another MRIclassification was published in 2020 [74],named the deep pelvic endometriosisindex (dPEI) classification,demonstrated a high reproducibility(kappa = 0,74), including the USL(Figure 5). This MRI classificationincludes the description of lateralcompartments and accurately predictsoperating time, hospital stay and postoperative complications [76]. Larger prospective European and American validation studies on the use MRI-based use of #ENZIAN and dPEI classifications are ongoing.
Statements on the use of imaging techniques for non-invasive diagnosis and classification of endometriosis
General statements
The test performance of any imaging technique for the detection of DE is operator dependent and will increase with exposure, level of training and skills and experience of the operator.
- Consensus: yes 96.2% (n=51); no 0% (n=0), abstain 3.8% (n=2)
Patients with a plan for surgical intervention for endometriosis should undergo pre- operative imaging for the detection of DE performed by adequately trained operators.
- Consensus: yes 96.2% (n=51); no 0% (n=0), abstain 3.8% (n=2)
Transvaginal sonography performed by adequately trained operators is recommended as first-line imaging tool due to its availability, good test performance, cost efficacy and its low environmental impact when compared to other imaging methods.
- Level of evidence: 1a
- Grade of statement: A
- Consensus: yes 96.2% (n=51); no 0% (n=0), abstain 3.8% (n=2)
Statements on ultrasonography
Imaging with TVS can reliably pre-operatively predict, and is recommended, to detect the presence DE of the rectum but is less accurate in sigmoidal DE due to limited visibility
- Level of evidence: 1a
- Grade of statement: A
- Consensus: yes 86.8% (n=46); no 5.7% (n=3), abstain 7.6% (n=4)
Imaging with TVS can help to pre-operatively predict the presence of DE of the rectovaginal septum
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 83.0% (n=44); no 3.8% (n=2), abstain 13.2% (n=7)
Imaging with TVS can help to pre-operatively predict the presence of DE of the vagina, uterosacral ligaments and parametrium
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 73.6% (n=39); no 18.9% (n=10), abstain 7.6% (n=4)
Imaging with TVS can help to pre-operatively predict the presence of DE of the bladder
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 90.6% (n=48); no 1.9% (n=1), abstain 7.6% (n=4)
Statements on MRI and CT
Imaging with MRI can reliably pre-operatively predict the presence of DE of the rectosigmoid
- Level of evidence: 1a
- Grade of statement: A
- Consensus: yes 90.6% (n=48); no 5.7% (n=3), abstain 3.8% (n=2)
Imaging with MRI can reliably pre-operatively predict the presence of DE of the uterosacral ligaments and torus uterinus
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 88.7% (n=47); no 0% (n=0), abstain 11.3% (n=6)
Imaging with MRI is helpful to pre-operatively predict the presence of DE of the rectovaginal septum
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 90.6% (n=48); no 3.8% (n=2), abstain 5.7% (n=3)
Imaging with MRI can reliably pre-operatively predict the presence of DE of the vagina
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 86.8% (n=46); no 3.8% (n=2), abstain 9.4% (n=5)
Imaging with MRI can reliably pre-operatively predict the presence DE of the bladder
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 92.5% (n=49); no 3.8% (n=2), abstain 3.8% (n=2)
Imaging with CT may reliably pre-operatively predict the presence of DE of the rectosigmoid but is less studied than other imaging modalities. There are, however, no obvious advantages compared to MRI as well as the disadvantage of radiation exposure.
- Level of evidence: 2a
- Grade of statement: B
- Consensus: yes 69.8% (n=37); no 22.6% (n=12), abstain 7.6% (n=4)
There is insufficient evidence to support, compared to other imaging modalities, for the use of CT for the detection of deep endometriosis of the uterosacral ligaments/torus uterinus, rectovaginal septum, vagina or bladder
- Level of evidence: 2a
- Grade of statement: D
- Consensus: yes 90.6% (n=48); no 1.9% (n=1), abstain 7.6% (n=4)
Statements on the non-invasive use of classification systems
Imaging with TVS in combination with the rASRM score can help describe moderate to severe endometriosis but is less accurate in cases of minimal to mild disease as classified with the rASRM score
- Level of evidence: 4
- Grade of statement: D
- Consensus: yes 62.3% (n=33); no 7.6% (n=4), abstain 30.2% (n=16)
Imaging with TVS and in combination with the (#)Enzian classification can reliably describe deep endometriosis, ovarian endometriosis and adhesions but is less accurate in cases of parametrial involvement (B compartment).
- Level of evidence: 1a
- Grade of statement: B
- Consensus: yes 83.0% (n=44); no 3.8% (n=2), abstain 13.2% (n=7)
Imaging with MRI and in combination with the (#)Enzian classification can reliably describe rectal and rectovaginal deep endometriosis, ovarian endometriosis but is less accurate in cases of USL and/or parametrial involvement (B compartment) and adhesions.
- Level of evidence: 4
- Grade of statement: B
- Consensus: yes 81.1% (n=43); no 5.7% (n=3), abstain 13.2% (n=7)
Imaging with TVS and in combination with the UBESS classification may help to estimate surgical complexity but the predictive value is not yet generalizable.
- Level of evidence: 3b
- Grade of statement: B
- Consensus: yes 64.2% (n=33); no 5.7% (n=3), abstain 30.2% (n=16)
Imaging alone with TVS and in combination with the EFI prediction cannot be reliably used as a substitute for the EFI generated by invasive, i.e. surgical methods.
- Level of evidence: 4
- Grade of statement: D
- Consensus: yes 62.3% (n=33); no 7.6% (n=4), abstain 30.2% (n=16)
Imaging alone with TVS in combination with the AAGL classification may be used as a substitute for the AAGL classification generated by invasive, i.e. surgical methods.
- Level of evidence: 2b
- Grade of statement: C
- Consensus: yes 50.9% (n=27); no 28.3% (n=15), abstain 20.8% (n=11)
Overview of consensus, discussion and conclusions
The present work represents a consensus opinion regarding use of imaging methods and non-invasive application of classification systems for the detection of DE, specifically when using TVS or MRI. The test performance of any imaging technique is operator dependent. Imaging with TVS and MRI needs to be performed by well- trained medical staff. TVS is recommended as first-line imaging tool due to its availability, good test performance, cost efficacy and its low environmental impact, although it is acknowledged that many centers adopt MRI as first line technique which is also appropriate.
There was strong agreement that TVS assessment of patients with suspected DE will accurately determine or rule out the presence of DE affecting the rectum, rectovaginal septum and bladder but is less precise in locations such as the parametrium and the uterosacral ligaments. However, the detection of DE of the uterosacral ligaments and parametrium using TVS is evolving and has been constantly improving. Similarly, MRI- based imaging is capable of detecting DE in these locations and a consensus was reached that MRI can reliably predict the presence of uterosacral ligament, parametrial and rectovaginal septum DE.
The use of classification systems for DE is a matter of constant debate. There was moderate agreement on the non-invasive use of rASRM, UBESS classification systems and EFI prediction model and equipoise on the usefulness of the TVS-based use of the AAGL score. The majority of participants strongly agreed on the use of TVS and MRI in combination with the (#)ENZIAN classification although it is less accurate in cases of parametrial und USL involvement. Future studies on rASRM, AAGL, UBESS, EFI and (#)ENZIAN will hopefully further clarify their future role in these settings.
It is noteworthy that the reference standards of many of the published studies have been laparoscopy, with/without histopathology. Hence, it is difficult to ascertain the limitation of operator expertise, or a reference standard which could be used in women who are managed conservatively. While this paper is focused on non-invasive imaging primarily for planning surgery, it is not the only aspect of endometriosis treatment, with at least 40% of women with DE being asymptomatic. In those with symptoms, it is not necessarily clear that these are caused by or coincide with endometriosis. Therefore, the statements made within this paper primarily pertain to women with symptomatic disease with a possible plan for surgical treatment. The combination assessment of women with potential DE with non-invasive imaging with TVS and/or MRI by adequately trained clinicians with planning of surgical and/or conservative management approaches should be the standard of care in health care facilities offering endometriosis therapy.
Acknowledgements, funding, disclosures
The authors thank AAGL, EEL, ESGE, ESHRE, ESUR, IDEA, ISGE and ISUOG, and their respective chairs, for their support. This consensus statement has been endorsed by the Arbeitsgemeinschaft Endometriose der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (AGEM of the DGGG), Arbeitsgemeinschaft Gynäkologische Endoskopie (AGE of the DGGG), Australasian Gynaecological Endoscopy & Surgery (AGES) Society, the Society of Endometriosis and Uterine Disorders (SEUD) and the Turkish Endometriosis & Adenomyosis Society.
The coordinating chairs (G.C., G.H.) wish also to express sincere gratitude to Hayley Moffit for organizing the teleconferences.
References
Supporting information on the internet
The following supporting information may be found in the online version of this article:
Figure S1 Revised American Society for Reproductive Medicine (rASRM) classification of endometriosis. Reprinted from the Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997; 67: 817–82149. Copyright © 1997 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved.
Figure S2 #Enzian classification system for women with superficial, ovarian and deep endometriosis. Reprinted from Keckstein et al.51, with permission from J. Keckstein. Copyright © 2021 The Authors. Published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG). Sacrouterine ligg/USL, uterosacral ligaments.
Figure S3 Ultrasound-based Endometriosis Staging System (UBESS), with sonographic features demonstrable on transvaginal ultrasound (TVS) and its prediction of level of surgical complexity. Adapted from Menakaya et al.52, with permission from ISUOG. SVG, sonovaginography.
Figure S4 Endometriosis fertility index (EFI) system. This score predicts fertility outcome for women who attempt non-in-vitro fertilization conception following surgically documented endometriosis. Reprinted from Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril 2010; 94: 1609–16157. Copyright © 2010 American Society for Reproductive Medicine, with permission from Elsevier. All rights reserved. AFS, American Fertility Society.
Figure S5 Magnetic resonance imaging (MRI) lexicon and deep pelvic endometriosis index (dPEI) classification: low extension (score 1 or 2), moderate extension (score 3 or 4) or severe extension (score 5 or more). Reproduced from Rousset P, Florin M, Bharwani N, Touboul C, Monroc M, Golfier F, Nougaret S, Thomassin-Naggara I, Group E. Deep pelvic infiltrating endometriosis: MRI consensus lexicon and compartment-based approach from the ENDOVALIRM group. Diagn Interv Imaging 2023; 104: 95–11266. Copyright © 2022 The Author(s). Published by Elsevier Masson SAS on behalf of Soci´et´e franc¸aise de radiologie. All rights reserved.
Table 1. Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for the detection of deep endometriosis of the rectosigmoid. DCBE, double contrast barium enema; CT, computed tomography; MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound. * Value calculated from the available study data
Table 2. Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for the detection of deep endometriosis of the uterosacral ligaments. MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound. * Value calculated from the available study data
Table 5. Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for the detection of deep endometriosis of the bladder. MRI, magnetic resonance imaging; TVS, transvaginal ultrasound. * Value calculated from the available study data
Table 3. Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for the detection of deep endometriosis of the rectovaginal septum. MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound. * Value calculated from the available study data
Table 4. Comparison of published meta-analyses on diagnostic accuracy of imaging modalities for the detection of deep endometriosis of the vagina. MRI, magnetic resonance imaging; RES, transrectal endoscopic sonography; TVS, transvaginal ultrasound. * Value calculated from the available study data
Appendix 1.
Identification of scientific evidence (literature research MEDLINE).
Appendix 2.
Levels of evidence and grades of statement used in this work. (Oxford Centre for Evidence-Based Medicine (CEBM) )
Appendix 3 (Figures)
Figure 1. The revised American Society for Reproductive Medicine classification of endometriosis [50].
Figure 2. The #ENZIAN staging system for women with deep endometriosis developed as a supplement to the revised American Society for Reproductive Medicine score, in order to provide detailed descriptions of the retroperitoneal structure. [52].
Figure 3. Ultrasound-based endometriosis staging system (UBESS) with sonographic features demonstrable on transvaginal ultrasound (TVS) and its prediction of level of surgical complexity [53].
Figure 4. Endometriosis fertility index (EFI) system. This score predicts the fertility outcome for women who attempt non- in vitro fertilization conception following surgically documented endometriosis [8].
Figure 5. MRI lexicon and deep pelvic endometriosis index (dPEI) classification. Low extension (score 1 or 2), Moderate extension (score 3 or 4) and severe extension (score 5 or more) [74].