Authors / metadata
Abstract
Purpose: To provide Saudi clinicians with guidelines to aide them in the conduct of diagnostic and operative hysteroscopy.
Methods: A panel of Saudi experts was put together in 2023 to produce the guideline. The Grading of Recommendations, Assessment, Development and Evaluation was used to approach to summarize the evidence and assess the quality.
Results: Findings based on the two uses of hysteroscopy: diagnostic and operative are presented. The goal is to provide clinicians with evidence-based recommendations to apply in different settings.
Conclusions: This guideline summarizes the recommendations based on the utilization of the hysteroscope. The recommendations were made by the panel based on a thorough review of the literature and their expert opinion.
Introduction
Hysteroscopy is an accurate and minimally invasive procedure used for direct visualization of the uterine cavity, endocervix, and vaginal canal. This technique serves both diagnostic and therapeutic purposes in gynecology (1). There have been a number of advances in the technology used to conduct hysteroscopy and the techniques utilized, thus making this procedure less painful and less invasive and allowing medical practitioners to use it in the office setting with minimal patient discomfort and substantial cost reductions (2). The great advantage of utilizing hysteroscopy in gynecology is that it has become a reliable method of diagnosis and treatment due to its accuracy and direct visualization. The only limitation for hysteroscopy is that it requires specific training and directed teaching with a considerable learning curve.
The new golden standard for hysteroscopy appears to be office hysteroscopy; rigorous research has confirmed this method to be safe, cost-effective, easy to apply, and allowing for faster recovery time. Another advantage of office hysteroscopy is that it could be used diagnostically and therapeutically at the same time – a “see and treat” option without the need for general anesthesia. Current recommendations call for the utilization of office hysteroscopy as the first-line option for both diagnosis and treatment.
The data on hysteroscopy in Saudi Arabia is limited. A study by Oraif in 2016 assessed patients’ perceptions of and their satisfaction with diagnostic hysteroscopy with endometrial biopsy conducted in an office setting as compared to a diagnostic hysteroscopy with dilation and curettage performed in the operating room (OR) (3). Women who underwent diagnostic hysteroscopy in the OR setting reported a lower pain score than women who underwent office hysteroscopy, although the mean pain score was quite low on a visual analogue scale (VAS) one to ten, and not different than pain that people experience daily. The office hysteroscopy group reached pre-operative fitness more quickly than the OR group, they did not need to recover from conscious sedation, they were able to return to work and regular activities, and to drive much earlier (3). A 2018 prospective study at King Fahd Hospital assessed uterine abnormalities in patients with repeated implantation failure (RIF) using a hysteroscope and reported that hysteroscopy could detect intrauterine pathologies which were missed by other investigative procedures (4). The objective of this guideline is to provide Saudi OBGYN clinicians with the proper recommendations that would aid them in the conduct of diagnostic and operative hysteroscopy while taking into consideration Saudi culture and patient preferences. This guideline has been developed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology (5) and relies on evidence from the literature. It has been crafted by a task force of Saudi experts using a multidisciplinary, collaborative approach to offer an updated and validated resource.
Instrumentation
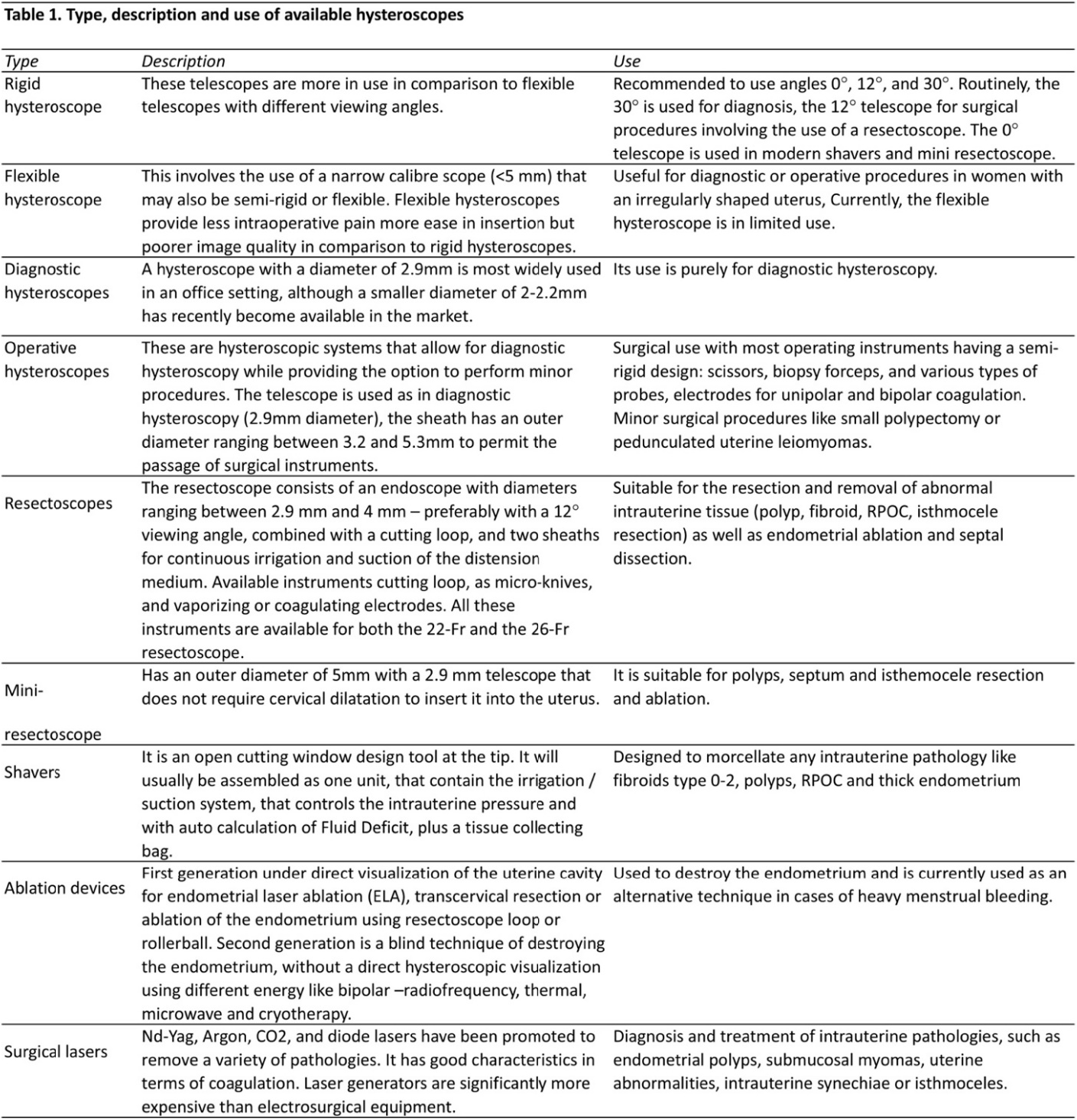
A diagnostic or operative hysteroscope differs in the outer diameters (OD) with a range between 2.2 to 10 mm, and its total outer diameter which refers to the sheath and usually ranges between 3.1 to 10 mm. The smaller the diameter of the sheath, the less pain and less need for cervical dilatation and preparation. The working length of a hysteroscope is measured from the eyepiece to the distal tip, and ranges between 160 to 302 mm; the longer the working length the more the person performing the hysteroscopy can reach away from the vagina. Both diagnostic and operative sheaths are fitted with stopcocks or ports for the instillation of distending media. To clear blood and thus improve visualization of the uterine cavity, some operative sheaths have dual ports that provide continuous laminar flow of distending media. In addition, some operative sheaths aspirate pieces of tissue from the uterine cavity. This allows removal of large debris while maintaining cervical dilation. Selected diagnostic hysteroscopes permit targeted biopsies and retrieval of foreign bodies, as well as limited intrauterine surgery. Advanced operative sheaths may have three channels: two for operative instruments such as biopsy instruments, forceps or scissors, and one for instilling distending media (6, 7).
Cold light source: A high-quality cold light source, preferably equipped with a Xenon or LED lamp, will usually yield the best results. A 175-watt light source is considered sufficient for routine procedures. A 300-watt light source is recommended for special applications or those performed with miniature telescopes. A light source operating at higher power levels usually produces more thermal energy, which in turn causes a greater rise in temperature. For standard hysteroscopic procedures, cold light cables with a diameter of 5 mm and a length of 180 cm are used.
Imaging systems: The use of an endo-camera is essential in modern hysteroscopy. Different types of video cameras are available, their quality depends on the following technical parameters: resolution, sensitivity (Lux), as well as the quality of the video images. A high signal-to-noise ratio is needed to assure high image quality under extreme situations such as in cases of hemorrhages. Modern High Definition (HD) cameras are recommended to offer a very high resolution and almost natural color reproduction. Video recorders and video printers are recommended, allowing still images, video and audio data to be recorded and archived.
Hysteroscopes: Hysteroscopes are available in many forms and their use varies depending on the clinical procedure while taking into consideration patient safety and comfort (Table 1).
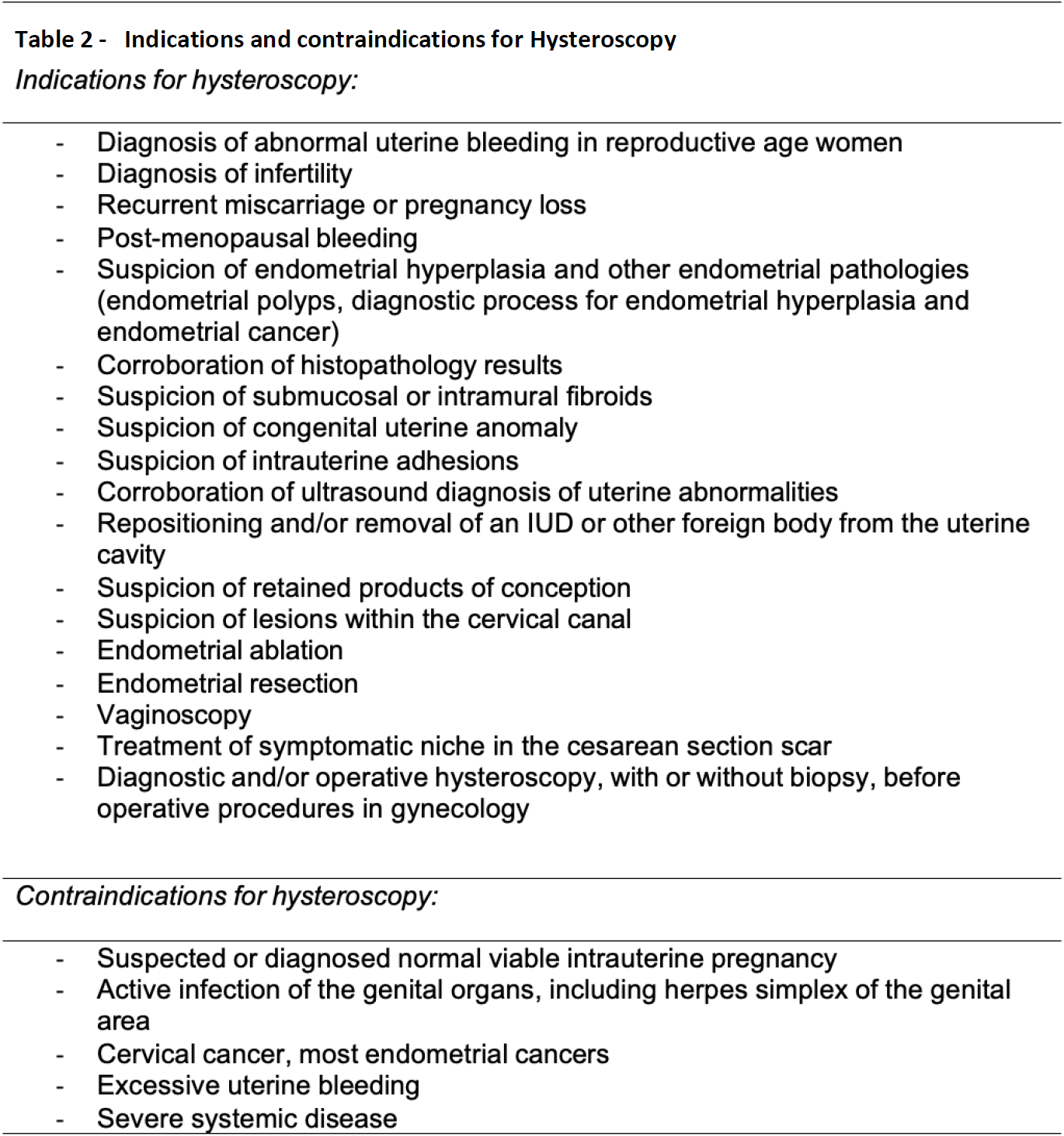
Implications for use: The safety and tolerability of hysteroscopy has rendered it a procedure with a wide range of possible applications, especially that it also allows the collection of tissue samples when needed. There are however some contraindications for hysteroscopy (Table 2).
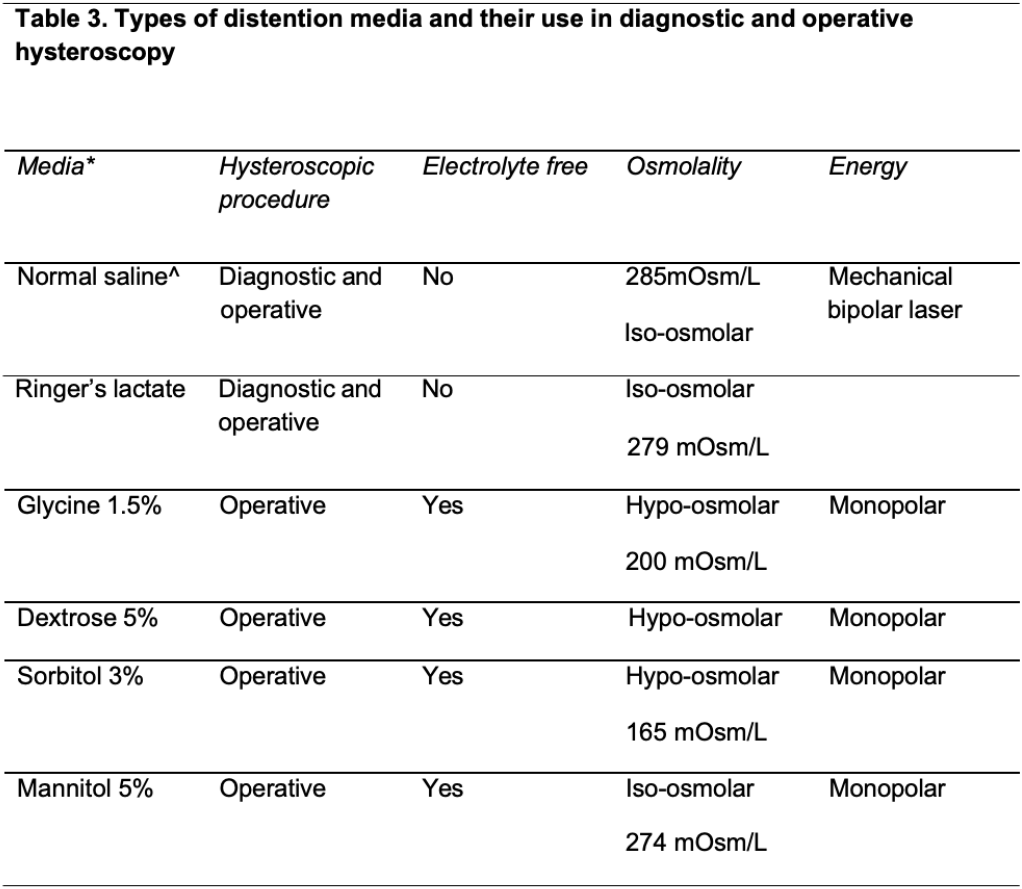
Distention media: Various distention media are utilized to expand the typically collapsed uterine cavity to enable the performance of diagnostic and operative hysteroscopic procedures. In an ideal scenario, the distention medium should provide clear visualization and be rapidly cleared from the body of the patient. It should also be non-toxic, non-hemolytic, isotonic, and less likely to cause allergic reaction. Distention media could largely be classified into fluid and gaseous categories.
Gaseous distension media: Gaseous distention media is not recommended for use in modern hysteroscopy(8).
Fluid distension: Fluid distension media could be subcategorized based on their viscosity, tonicity, and electrolyte content (8). Normal saline is the safest and most frequently used electrolyte-rich medium in modern-day diagnostic and operative hysteroscopy. The isotonic nature and “physiologic” electrolyte content of this medium would largely eliminate the risk for electrolyte imbalance even if a significant amount of normal saline is absorbed during hysteroscopy. Nonetheless, absorption and/or intraperitoneal spillage of large amounts of electrolyte rich fluid could also lead to complications related to hypervolemia like pulmonary oedema and heart failure (8).
High viscosity distention media: High viscosity distention media is not recommended for use in modern hysteroscopy.
Low viscosity distention media: Low viscosity distention media could be further categorized into electrolyte-rich and electrolyte-poor media. Normal saline is the safest and most frequently used electrolyte-rich medium in hysteroscopy today. Electrolyte-poor media, which include 5% mannitol, 3% sorbitol, and 1.5% glycine, allow the use of monopolar electrosurgical hysteroscopes. When absorbed or spilled into the peritoneal cavity, however, these could cause a significant drop in serum osmolality and lead to life-threatening hyponatremic hypervolemia (9). The development of bipolar electrosurgical hysteroscopes has largely confined the use of such solutions to diagnostic hysteroscopy and to some operative hysteroscopic procedures where bipolar electrosurgical or mechanical tissue removal system is not available (Table 3).
Maintenance of appropriate intrauterine pressure: It is essential to establish and maintain a sufficient intrauterine pressure using the distention medium to expand and visualize the usually collapsed endometrial cavity and suppress the occasional endometrial bleeding to ensure a successful hysteroscopic procedure. A starting pressure of 70-80 mmHg is recommended and can later be carefully raised according to the need of the surgeon for a specified period rather than the entire duration of the procedure.
Fluid deficit monitoring: Accurate and frequent monitoring – every 10 minutes after initiation of the procedure – of the excess fluid absorption is a crucial step to conduct safe hysteroscopic procedures, especially in the more complex and prolonged operative cases, as early recognition of fluid overload enables the team to evade possible complications. This is achieved by the measurement of all the fluid remaining in the used fluid bags and fluid collected from the outflow channel and leaked from the cervix into the bucket, surgical drapes and the floor. The sum is then subtracted from the total amount of fluid infused into the uterus to get the amount of fluid that leaked into the patient’s circulation or peritoneal cavity. It is recommended to use an automated fluid management system which can calculate the exact weight of infused fluids and fluids leaked and come up with an accurate and more precise estimation of the fluid deficit in a continuous fashion.
Fluid overload: Despite the lack of robust evidence to support the determination of cutoff values to define fluid overload, several entities have defined it as a fluid deficit of more than 1000 ml when a hypotonic distention medium and more than 2500 ml when an isotonic distention medium is used in a healthy young patient (8). A lower threshold should be implemented in older patients and in patients with significant co-morbidities.
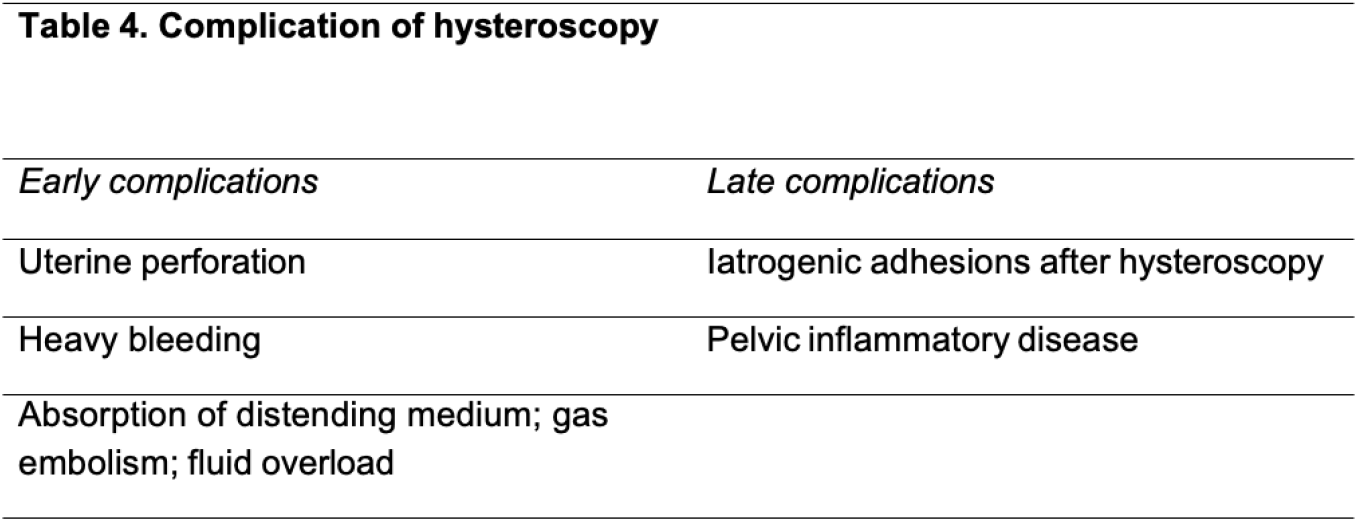
Complications of hysteroscopy: Complications associated with hysteroscopy can be identified early on during the procedure or later after the procedure has been finalized and the patient has been discharged (Table 4).
Methodology
Committee members: A committee of seven Saudi experts, mainly gynecologists came together on several occasions to outline this guideline. The group also included patient representatives, and a Saudi Ministry of Health representative. The committee initially determined the questions of interest. The questions were structured using PICO (P: patient, I: Intervention, C: comparison, O: Outcome) methodology. The outcomes of interest for our guideline included diagnostic or operative hysteroscopy techniques, medications, media used, equipment, anesthesia methods, quality of life, any adverse events, and cost.
Search strategy: A thorough search of the literature for the available systematic reviews and meta-analyses in PubMed, Embase, MEDLINE and Cochrane databases was conducted. The search was conducted on June 21, 2024. Keywords used included: hysteroscopy, operative, diagnostic, AUB, sedation, timing, medium, isthmocele, adhesion, fertility, myomectomy, MRI, endometrial sampling, sonogram, implantation failure. The search was limited to articles in English. We also conducted systematic searches for contextual information necessary to develop the full guideline for Saudi Arabia, including searches for information on patients’ values and preferences, and costs and resource use specific to the Saudi setting.
Evidence to decision: The GRADE approach for each PICO question was observed, and evidence profiles and tables were developed (5). The information was then shared with the panelists and any additional information or input from panel members was encouraged.
Quality of the evidence: The GRADE working group defines the quality of evidence as the degree of confidence that the estimate of an effect is adequate to support a particular decision, or recommendation (5). The quality of evidence using the GRADE approach was assessed. There are four classifications for the quality of evidence, ranging from high to low. The decision regarding the quality is based on panelists’ decisions on methodological aspects of the collected evidence. Evidence that is rated high means that the panelists are very confident that the true effect lies close to that of the estimate of the effect. Moderately rated evidence means panelists are moderately confident in the effect estimate, and that there is a possibility that the true effect and the estimate of the effect are substantially different. Evidence that is rated low means that the confidence in the effect estimate is limited, whereas evidence rated as very low means that there is very little confidence in the effect estimate. In the absence of any conclusive scientific evidence, some practices have nevertheless been recommended based on agreement between all members of the expert panel (“expert opinion”).
Recommendations: The evidence was summarized and presented during meetings and online. Discussions regarding the evidence and the recommendations that ensued followed an evidence-to-decision process as detailed in the GRADE methodology, thus allowing proper documentation and deliberation until a consensus was reached. The recommendations were graded and labelled as strong or weak. When the recommendations were strong the committee formulated their recommendations using “we recommend” or “clinicians should” to guide the application of the recommendation and indicate that it should be used for most patients. When the recommendation was weak, the committee’s recommendation used wording to “suggest” and maintain that the recommendation requires further decision- making and discussion.
Target audience: Our target audience is clinicians, particularly OBGYN clinicians who practice in the Kingdom of Saudi Arabia. Policy makers can also benefit from these recommendations and can refer to them. It is important to remember that when implementing these guidelines, all physicians should utilize their own discretion, considering their individual expertise and the unique characteristics of their practice or institution. This ensures the selection of the most appropriate diagnostic or treatment approach tailored to each particular patient.
Diagnostic Hysteroscopy
Diagnostic hysteroscopy, an investigation that is frequently performed in the outpatient setting, is generally safe and brief. Most women can undertake the procedure with or without local anesthesia and find it both convenient and acceptable. Diagnostic hysteroscopy is a valuable tool in obstetrics and gynecology for direct imaging of the contents of the uterus and is commonly used to examine and detect visualization irregularities in the uterine cavity.
Should office hysteroscopy vs. operative room be used in diagnostic hysteroscopy? The use of office hysteroscopy has been well established for the diagnostic evaluation of abnormal uterine bleeding (11). The selection of patients for office-based hysteroscopic procedures depends on the physician’s understanding of the suspected pathology, lesion size, lesion depth, patients’ readiness to undergo an office-based procedure, physicians’ skills and expertise, a thorough assessment of patient comorbidities, and availability of appropriate equipment and patient care. Considerations for performing hysteroscopy in an alternative setting, such as the operating room or ambulatory surgery, ought to be considered for patients who have anxiety or have not tolerated the office-based approach in previous procedures (11). A 20-year-retrospective study, with data from 2675 patients who had vaginoscopic office hysteroscopy, reported hysteroscopy as an efficient and safe mode to investigate pathologies within the uterus and reported an overall patient satisfaction with the process (12). The study compared results from hysteroscopic findings with the respective histology reports to reveal that in cases of normal endometrium, a sensitivity of 60.9%, specificity of 92.1%, Positive Predictive Value (PPV) of 79.07%, and Negative Predictive Value (NPV) 82.8% were estimated. A systematic review by Bennett et al. in 2019 found no studies that compared the diagnostic accuracy of outpatient hysteroscopy with hysteroscopy performed in the operating room. However, they did conclude that outpatient hysteroscopy is significantly less expensive than operating-room hysteroscopy (13). In general, studies have reported a patient preference for office-based hysteroscopy due to higher patient satisfaction with the procedure and faster recovery. Other possible benefits of office hysteroscopy include patient and physician convenience, avoidance of general anesthesia, less patient anxiety owing to office setting familiarity, cost-effectiveness, and more efficient use of the operating room for more complex hysteroscopic cases (14-16).
Recommendations
Recommendation 1: The panel recommends the use of outpatient hysteroscopy for diagnostic procedures taking into consideration patient comorbidities, patient acceptability, availability of proper equipment, and physician skills and expertise (strong recommendation, high quality evidence).
Should conscious sedation vs. no sedation be used in office diagnostic hysteroscopy? Pain management during office hysteroscopy could be required, whether the procedure is for diagnostic or operative purposes and due to the utilization of instruments in the genital tract, distention of the uterine cavity and in cases of operative hysteroscopy due to procedural interventions such as biopsy (17, 18). A systematic review and meta-analysis by De Silva et al. in 2021 reported two studies assessing the use of conscious sedation during diagnostic hysteroscopy(19). In both studies intravenous conscious sedation did not show any benefit when compared to other forms of sedation, and in fact, caused increased pain, both during and after hysteroscopy in one of the studies (20, 21). According to the Royal College of Obstetrics and Gynecology, conscious sedation should not be routinely used in outpatient hysteroscopic procedures as it has no advantage in terms of pain control and patient satisfaction as compared to local anesthesia (22). The utilization of conscious sedation could also cause life-threatening complications and thus if it were to be used appropriate monitoring and proper staff skills are mandatory (22).
Recommendation 2: The panel recommends the limited use of conscious sedation in outpatient diagnostic hysteroscopy as it has no benefit in terms of pain control and patient’s satisfaction (strong recommendation, moderate quality evidence).
The panel recommends mandatory monitoring and appropriate staff skills if diagnostic hysteroscopy will be conducted under conscious sedation according to the institutional policies and facilities available (strong recommendation, moderate quality evidence).
Should a specific timing relative to the menstrual cycle vs. any time impact success of diagnostic hysteroscopy? Our search of the literature returned no studies evaluating the optimal timing of hysteroscopy. Most guidelines and publications describe the optimal timing for diagnostic hysteroscopy, in premenopausal women with regular menstrual cycles, during the follicular phase of the menstrual cycle after menstruation. Pregnancy should be reasonably excluded before performing hysteroscopy. Hysteroscopy during the secretory phase of the cycle may make diagnosis more difficult because a thickened endometrium may mimic polyps (11, 23, 24). A systematic review in 2007 selected studies that only performed hysteroscopy in the follicular phase of menstruation. Their findings did not show any clinical significance and therefore no evidence-based recommendation on the subject of best timing could be made (25).
Recommendation 3: The panel recommends the follicular phase as the optimal timing for diagnostic hysteroscopy, after menstruation, in premenopausal women (strong recommendation, moderate quality evidence).
Should initial hysteroscopy vs. ultrasound be used to diagnose etiology of AUB?
Abnormal uterine bleeding (AUB) in women is the single most common reason for gynecological referrals. In more than 40% of the referred patients, polyps and myomas have been reported (26). Pelvic ultrasound has been used to evaluate the uterine cavity for fibroids, endometrial thickness, endometrial homogeneity, and the presence of abnormal vascularity within the endometrium. A systematic review by Van Dongen et al, conducted to assess the accuracy and feasibility of diagnostic hysteroscopy in the evaluation of intrauterine abnormalities in women with AUB, revealed that diagnostic hysteroscopy is both accurate and feasible in the diagnosis of intrauterine abnormalities (25). Another systematic review to determine the accuracy of transvaginal ultrasonography (TVUS), sono hysterography and diagnostic hysteroscopy for the investigation of AUB in premenopausal women, included prospective studies and suggested that TVUS has a higher rate of false negatives for detecting intrauterine pathology compared with diagnostic hysteroscopy. Diagnostic hysteroscopy has excellent diagnostic accuracy for diagnosing submucous fibroids; and performed best when detecting submucous fibroids (27).
Recommendation 4: The panel recommends an evaluation plan using transvaginal sonography as the initial screening evaluation, followed by hysteroscopy when needed (strong recommendation, moderate quality evidence).
The panel suggests the use of diagnostic hysteroscopy for the diagnosis of AUB (conditional recommendation, moderate quality evidence).
Should hysteroscopy vs. MRI be used to diagnose etiology of AUB? Magnetic resonance imaging (MRI), when indicated, is an excellent second-line diagnostic tool for a better non-invasive characterization of the underlying cause of AUB (28). No randomized controlled trials comparing the diagnostic efficacy of hysteroscopy versus MRI for AUB were retrieved. An observational study reported findings from patients who presented with AUB and were referred to the department of radiodiagnosis and underwent USG of the abdomen and pelvis, followed by an MRI of the pelvis. The study analyzed and compared the two methods with the histopathological examination (HPE) of the samples of hysterectomized uterus, polypectomy, myomectomy, and dilation and curettage (D&C) of the endometrium. Among the study population, USG reports showed two patients (4.10%) with polyps, seven patients (14.58%) with adenomyosis, 25 patients (52.08%) with leiomyomas, and 14 patients (29.16%) with malignancies. On MRI examination, three patients (6.25%) were diagnosed with polyps, nine patients (18.7%) with adenomyosis, 22 patients (45.8%) with leiomyomas, and 14 patients (29.16%) were reported to have malignancies. The measure of agreement with the kappa value for MRI and HPE in evaluating the causes of AUB is 1.0 (very good). Whereas the kappa agreement value of USG and HPE in evaluating the causes of abnormal uterine bleeding is 0.903 (acceptable). The sensitivity of USG in diagnosing polyps, adenomyosis, leiomyoma, and malignancy was observed at 66%, 77.78%, 100%, and 100%, respectively. The sensitivity of MRI in diagnosing polyps, adenomyosis, leiomyoma, and malignancy was 100% for each. The study concluded that an MRI is the most effective method for accurate identification of the location, number, and characterization of lesions, extensions, and staging of carcinomas (29).
Recommendation 5: The panel suggests the use of MRI for diagnosis of AUB only when further investigations are warranted (conditional recommendation, low quality evidence).
Should hysteroscopy vs. SIS be used to diagnose etiology of AUB? Saline infusion sonogram (SIS) involves the instillation of sterile saline, a negative contrast agent, into the uterus through a hysterosalpingography catheter prior to TVUS. Compared to TVUS, SIS has been reported in premenopausal women to allow easier differentiation of polyps, submucous fibroids, and endometrial lesions that emerge clearly in anechoic saline (30). SIS is accurate in the evaluation of the uterine cavity in pre- and postmenopausal women suffering from AUB. The feasibility of saline contrast hysterosonography is high, although significantly better in premenopausal women compared with postmenopausal women (31).
Recommendation 6: The panel suggests the use of SIS as an initial investigation to diagnose the etiology of AUB, before resorting to hysteroscopy when needed (conditional recommendation, moderate quality evidence).
Should hysteroscopy vs. endometrial sampling be used to diagnose etiology of AUB? Among the most frequent indications for endometrial biopsy (EB) in clinical practice include infertility and subfertility, the assessment of the uterine cavity before assisted reproduction technique (ART); and the evaluation of premenopausal and postmenopausal patients with AUB among others (32). A 2015 systematic review, assessing the accuracy of endometrial sampling for the diagnoses of endometrial cancer, atypical hyperplasia and endometrial disease (endometrial pathology, including benign polyps), searched the literature for studies comparing the results of endometrial sampling in women with postmenopausal bleeding with two different reference standards: blind dilatation and curettage (D&C) and hysteroscopy with histology. A total of 12 studies were detected, reporting on 1029 women with postmenopausal bleeding: five studies with D&C and seven studies with hysteroscopy as a reference test. The weighted sensitivity of endometrial sampling with D&C as a reference for the diagnosis of endometrial cancer was 100% (range 100-100%) and 92% (71-100) for the diagnosis of atypical hyperplasia. Only one study reported sensitivity for endometrial disease, which was 76%. When hysteroscopy was used as a reference, weighted sensitivities of endometrial sampling were 90% (range 50-100), 82% (range 56-94) and 39% (21-69) for the diagnosis of endometrial cancer, atypical hyperplasia and endometrial disease, respectively. For all diagnoses studied and the reference test used, specificity was 98-100%. The weighted failure rate of endometrial sampling was 11% (range 1-53%), while insufficient samples were found in 31% (range 7-76%). In these women with insufficient or failed samples, an endometrial (pre) cancer was found in 7% (range 0-18%). In women with postmenopausal bleeding, the sensitivity of endometrial sampling to detect endometrial cancer, atypical hyperplasia and endometrial disease, including endometrial polyps, is lower than previously thought (33).
Recommendation 7: The panel recommends office hysteroscopy for targeted biopsy due to its high diagnostic accuracy and cost-effectiveness (strong recommendation, moderate quality evidence).
The panel recommends EB in postmenopausal women with any kind of AUB or PMB (strong recommendation, high quality evidence).
Should hysteroscopy be used in recurrent implantation failure (RIF)? It has been recently proposed that office hysteroscopy be used for the assessment and possible management of infertility since it is a golden tool for screening intracavity lesions. In 2013, Cenksoy et al. reported that 44.9% of RIF patients had abnormal hysteroscopic findings preceding their IVF cycle, 48.1% became pregnant after hysteroscopy and nearly half of these pregnant women had uterine abnormalities corrected. They found that the implantation rate and CPR were statistically significantly increased after polypectomy and concluded that hysteroscopy has positive prognostic value for patients who experience RIF (34). A meta-analysis by Cao et al reported that hysteroscopy prior to an IVF cycle significantly improves outcomes for patients with RIF, particularly in Asia. These results indicate that visual assessment of uterine morphology by OH with or without corrected abnormalities may be of positive prognostic value for achieving a pregnancy outcome in patients with RIF (35). Another more recent systematic review by Vitale et al in 2023 revealed that the evidence regarding the role of hysteroscopy in boosting fertility in women undergoing ART is evolving through new trials. The evidence shows that diagnostic hysteroscopy substantially improves LBR of patients with at least one failed implantation after embryo transfer. When evaluating CPR instead of LBR, there is moderate-quality evidence showing that performing hysteroscopy before ART improves outcomes, even of women without a history of unsuccessful implantation (36). The role of office hysteroscopy in assisted reproductive techniques remains controversial, and additional studies are needed to determine if hysteroscopic resection of endometrial polyps has an impact on fertility outcomes.
Recommendation 8: The panel suggests the use of office hysteroscopy in recurrent implantation failure to detect uterine abnormalities after preliminary uterine assessment (conditional recommendation, moderate quality evidence).
Operative Hysteroscopy
Operative hysteroscopy allows for the ability to treat any observed intrauterine pathology. There is significant overlap in indications for diagnostic and operative procedures and if a procedure is started with diagnostic intent, the “see and treat” approach can be used (37). This approach allows for a seamless transition from diagnostic to operative hysteroscopy if abnormal pathology is noted and if the patient continues to tolerate the procedure. Assuming proper set-up and instrumentation availability, this technique allows for the fewest number of interventions for proper patient care. Gynecologists perform operative hysteroscopy to manage intrauterine conditions like endometrial polyps, uterine fibroids, uterine septa, retained products of conception and adhesions. Other applications include removing foreign objects such as displaced intrauterine devices, performing tubal cannulation, treating isthmocele, and conducting targeted biopsies (11).
Should we use normal saline vs glycine in operative hysteroscopy? Normal saline is often recommended as the distention medium for operative hysteroscopy procedures because it enhances image clarity and reduces the frequency of vasovagal episodes when compared to carbon dioxide (38). Although the distension of the myometrial wall with saline commonly leads to contractions that patients report as colicky pain of moderate to severe intensity (39). Pain has been reported as the major reason leading to unsuccessful office hysteroscopic interventions (40). Reducing pain and discomfort is therefore a main concern during hysteroscopic procedures. Our search retrieved no RCTs assessing the use of glycine for operative hysteroscopy. A systematic review and meta-analysis by Baradwan et al, looked at RCTs to assess effectiveness of warmed saline distension medium in the intervention group versus room temperature distension medium in the control group among women undergoing diagnostic and/or operative office hysteroscopy. The review encompassed five RCTs that met the inclusion criteria with a total number of 441 patients. The study found that warm saline was linked to a significant reduction in the VAS pain score during the procedure compared to the control group (mean difference (MD) = -1.12, 95% confidence interval (CI) (-1.80, -0.45), p = 0.001). Moreover, the VAS pain score after the procedure was significantly declined among the warm saline group (MD = -0.62, 95% CI (-0.97, -0.27), p = 0.005). Interestingly, more patients were significantly satisfied with warm saline distension medium application compared to room temperature group (odds ratio (OR) = 3.71, 95% CI (2.01, 6.86), p< 0.001) (41). Another systematic review assessed the impact of distension medium type, pressure, and temperature for both diagnostic and operative office hysteroscopy, primarily on pain, but also on procedural success and duration, image quality, complications, and satisfaction and/or acceptability from both the patient’s and operator’s perspectives. Normal saline should be the preferred distension medium for office hysteroscopy. It allows for the efficient practice of “see and treat” services, in which diagnosis can be immediately followed by treatment. It is isotonic, minimizing risks associated with fluid overload, and is able to conduct electricity, essential for the operation of modern, miniature bipolar electrosurgical electrodes and works optimally with modern mechanical tissue removal systems (42).
Recommendation 9: The panel recommends the use of normal saline distention media for the conduct of office operative hysteroscopy (strong recommendation, moderate quality evidence).
Should office setting vs. operative room (OR) setting be used in operative hysteroscopy? Should office vs. operative hysteroscopy be used for polypectomy? There is a current trend towards shifting the implementation of hysteroscopies from the operating room to the office setting. Office procedures are associated with higher patient satisfaction and faster recovery. Other potential benefits of office hysteroscopy include patient and physician convenience, avoidance of general anesthesia, less patient anxiety related to familiarity with the office setting, cost effectiveness, and more efficient use of the operating room for more complex hysteroscopic cases (11). Hysteroscopic polypectomy is effective and safe as both a diagnostic and therapeutic intervention. There are a variety of methods practiced to remove polyps with hysteroscopy; however, there are no comparative studies for these methods with regards to efficacy or costs, and the method of choice is the one with which the clinician is trained in and most familiar, given that such technology is available. Office hysteroscopic polypectomy has been shown to be safe, well tolerated, and more cost effective compared with traditional inpatient hysteroscopic polypectomy. A pragmatic multicenter randomized controlled non-inferiority study to compare the effectiveness and acceptability of outpatient polypectomy with inpatient polypectomy was conducted in 31 UK National Health Service hospitals. Participants were randomly assigned to either outpatient uterine polypectomy under local anaesthetic or inpatient uterine polypectomy under general anesthesia. 73% (166/228) of women in the outpatient group and 80% (168/211) in the inpatient group reported successful treatment at six months (intention to treat relative risk 0.91, 95% confidence interval 0.82-1.02; per protocol relative risk 0.92, 0.82-1.02). Failure to remove polyps was higher (19% v 7%; relative risk 2.5, 1.5 to 4.1) and acceptability of the procedure was lower (83% v 92%; 0.90, 0.84 to 0.97) in the outpatient group. The authors reported that the quality of life did not differ significantly between the groups. Four uterine perforations, one of which necessitated bowel resection, all occurred in the inpatient group. Outpatient polypectomy was non-inferior to inpatient polypectomy. Failure to remove a uterine polyp was, however, more likely with outpatient polypectomy and acceptability of the procedure was slightly lower (43).
Recommendation 10: The panel suggests the use of outpatient hysteroscopy for operative procedures taking into consideration patient comorbidities, patient support, availability of proper equipment, and physician skills and expertise (conditional recommendation, high quality evidence).
The panel suggests the use of office hysteroscopy for the treatment of endometrial polyps whenever possible with a thorough counselling of patient on risks and complications (conditional recommenddation, moderate quality evidence).
Should tissue removal system vs resectoscopes be used in polypectomy? Traditional hysteroscopic resectoscopes utilize the loop electrode to resect polyps, and also electro coagulate the base to stop bleeding. The postoperative recurrence rate is low, so it is widely used in clinical practice. A meta-analysis in 2022 comparing the efficacy of hysteroscopic morcellation to resectoscopy in treatment of patients with endometrial lesions, revealed no statistically significant difference in the surgical success rate between the two groups in the management of endometrial lesions, and there was no statistically significant difference between the two in terms of body fluid deficit either. The study concluded however that hysteroscopic morcellation has better accuracy, effectiveness, and safety. Hysteroscopic morcellation will not cause electrical damage to the patient, no scar formation is caused by heat injury, can better protect the patient’s endometrium, can maximize the protection of the remaining normal endometrium and promote its movement, to promote the recovery of the endometrium and uterine cavity shape. This surgical method has fewer postoperative complications and better safety. Compared with traditional electrosurgical resection, hysteroscopic morcellation requires a shorter learning time and it has a shorter learning curve (44). These two treatment modalities have clinical applications, but both have their own shortcomings. While studies have shown the feasibility of this new surgical approach, individual studies lack sufficient capacity to provide accurate estimates due to small sample sizes in terms of surgical success rate, duration of surgery, and patient acceptability. In addition, the effectiveness and safety of the technology also need to be considered. According to the AAGL guidelines for the management of polyps, there does not appear to be differences in clinical outcomes with different hysteroscopic polypectomy techniques (45).
Recommendation 11: The panel commends the use of tissue removal system for polypectomy (strong recommendation, moderate quality evidence).
The panel suggests that clinicians carefully assess patient’s symptoms and provide thorough counselling to choose the most effective and safe modality for polypectomy (conditional recommendation, moderate quality evidence).
Should resectoscope, laser, or tissue collection system be used in retained product of conception (RPOC)? Benign intrauterine lesions include mainly endometrial polyps, retained products of conception (RPOC), and submucous leiomyoma, which are commonly seen in women of reproductive age. RPOC refers to placental or fetal tissue that can occur with induced abortion during early-term pregnancy, induction of labor during mid-term pregnancy, drug-induced abortion, miscarriage, Cesarean delivery, or full-term normal delivery. Patients often manifest with abnormal uterine bleeding after delivery, which predisposes them to infection and can even affect their fertility (46). To be more specific, the discussion of hysteroscopy resection of RPOC herein is meant for any RPOC in situ for 4-6 weeks. For almost a century, dilatation and blind removal through sharp, blunt, or suction has been used to surgically treat RPOC. However, blind techniques are associated with complications such as heavy bleeding, infections, and uterine perforation. Moreover, persistent RPOC can occur after a blind D&C. In addition, a common adverse outcome associated with D&C is intrauterine adhesion (IUA) formation.
Goldenberg in 1995 was the first to report the use of hysteroscopy for RPOC, facilitating directed identification and treatment. Over the last 10 years, several studies have been conducted to evaluate the efficacy of hysteroscopy for the treatment of RPOC (47). Lasers represent an alternative energy source to electrosurgery that is gaining interest in gynecologic surgery (48). Several types of lasers have been used in the gynecologic field: the Nd-Yag laser, Argon laser, CO2 laser, and the recent diode laser. Unfortunately, our search returned no literature assessing the use of lasers for RPOCs.
A systematic review by Vitale et al in 2021 to analyze the effect of hysteroscopic management of RPOC and analyze its effect on surgical and reproductive outcomes reported that the hysteroscopic approach to the patient diagnosed with RPOC is effective and safe, completely resecting the pathologic condition in a single procedure in 91% of the cases, and having low rates of complications, infection, and IUA formation. Moreover, women who tried to conceive after the procedure had a high rate of fertility and live births, with a low rate of subsequent pregnancy loss (49). A study comparing the clinical efficacy of hysteroscopic tissue removal system (TRS) and hysteroscopic electro resection in the treatment of benign intrauterine lesions, concluded that TRS has advantages of a shortened operative time and improvement in reproductive outcomes such as pregnancy rate (46). To date, there is no agreed-upon standard approach to RPOC. Blind D&C is still the most widely used first-line method of managing ultrasound-diagnosed RPOC, but not without risks. The hysteroscopic technique, with targeted removal of the pathologic condition, minimizes trauma to the healthy endometrium, with both short- and long-term potential benefits. The safety and feasibility of hysteroscopic resection, relative to blind D&C, are increasingly accepted for most forms of intrauterine pathologic conditions. Vitale et al found RPOC leading to IUA formation after hysteroscopic management in only 9 cases out of 1323 procedures (0.07%). These findings suggest the rarity of creating adhesions after hysteroscopic cold loop resection or mechanical removal of RPOC. In fact, the method of pathologic condition removal may influence the risk of de novo adhesions (49).
Recommendation 12: The panel recommends the use of operative hysteroscopy for the management of RPOC at least 4-6 weeks from miscarriage (strong recommendation, moderate quality evidence). The panel suggests the use of TRS for the management of RPOCs but only after a thorough evaluation of the patient before the procedure (conditional recommendation, moderate quality evidence).
Should a resectoscope be used in treatment of uterine septum in recurrent foetal loss? Septate uterus is a congenital malformation characterized by the failure of resorption of the tissue connecting the two paramesonephric ducts before the 20th embryonic week. Although uterine anomalies were described back in the 1800s by Cruveilhier and Von Rokitansky, there is still no consensus between different societies for the definition of septate uterus (50). Septate uterus is associated with adverse fertility outcomes, a lower natural conception, higher first-trimester miscarriage rate, adverse pregnancy outcomes (preterm delivery < 37 weeks), intrauterine growth restriction, and adverse obstetrical outcomes (malpresentation at delivery and perinatal mortality) compared to a control group (50). According to a meta-analysis conducted in 2024, comparing every method used for hysteroscopic septoplasty in a network meta-analysis, the use of scissors with this technique has significantly higher clinical pregnancy rate in comparison to resectoscope. As far as miscarriage rate and live birth rate are concerned, no significant differences have been found between the different techniques. Although the risk of adhesion formation after hysteroscopic septoplasty is believed to be low, diverse treatment options such as antibiotics, estrogen, intrauterine balloon or device for the postoperative period have been proposed. According to the ASRM guideline for the treatment of septate uterus, it is recommended to counsel patients with infertility and/or undergoing fertility treatment that resection of septum may or may not be associated with an increase in live births. Given limitations in the literature and the low risk of the procedure, septum incision may be offered to patients in a shared decision-making model.
Recommendation 13: The panel suggests septum incision using hysteroscopy in patients with a septum and a history of recurrent miscarriage with proper patient counselling (conditional recommendation, moderate quality evidence).
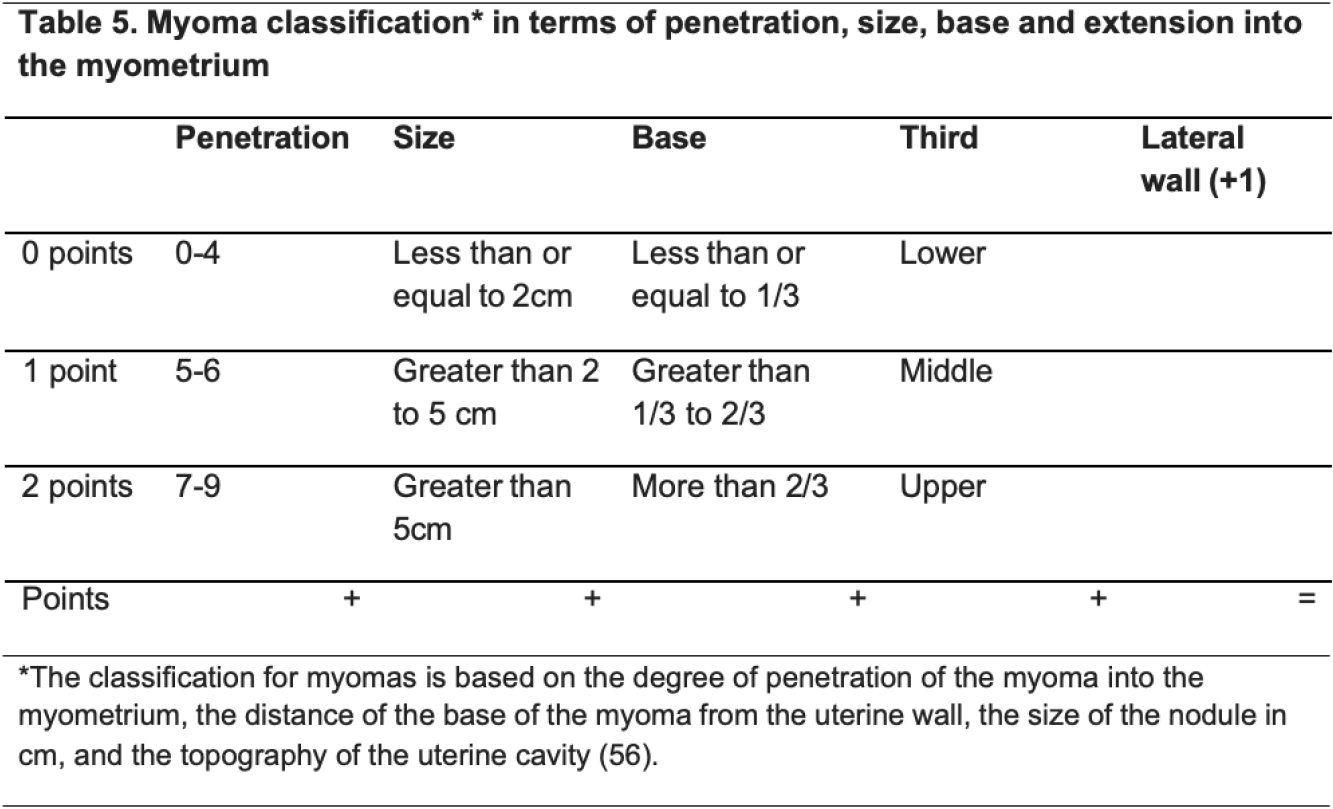
Should resectoscope, shaver or laser be used in myomectomy? Hysteroscopic myomectomy is widely used for the treatment of abnormal uterine bleeding in the setting of submucosal uterine leiomyoma (51). Uterine leiomyomas are benign monoclonal smooth muscle cell tumors of the myometrium and represent the most common pathology of the female genital tract. Although most myomas are asymptomatic, some, depending on their location, size, and number, can be responsible for pelvic pain, abnormal uterine bleeding, and states of subfertility and infertility (Table 5) (48). Hysteroscopic myomectomy is considered the first-line treatment option in the surgical management of submucosal fibroids, and double-flow bipolar resectoscope remains the gold-standard surgical device to approach submucous myomas (11, 52). The reported complication rate for hysteroscopic myomectomy ranges between 1% and 12%, with rates of 1–5% reported in most studies. The success of hysteroscopic myomectomy is dependent on the type of submucosal leiomyoma and one of the main disadvantages associated with resectoscopic myomectomy is the removal of healthy endometrium and myometrium with extensive thermal damage caused by electrosurgical instruments (11). Etrusco et al in 2023 conducted a systematic review to evaluate the use of diode laser for “see- and-treat” hysteroscopy in the management of intrauterine pathology. Eight studies were included in the qualitative analysis for a total of 474 patients undergoing laser hysteroscopic surgery. Except for leiomyomas, which were already planned for a two-phase intervention, only seven patients required a second surgical step. Cumulative rates of intraoperative and postoperative complications of 2.7% and 0.6%, respectively, were reported (48). The use of hysteroscopic TRS or intrauterine shavers has the benefit of avoiding approaches based on energy thanks to mechanical morcellation and instant aspiration of the tissue. Most studies have reported the benefits of hysteroscopic tissue removal systems such as shorter operative time, higher total resection rate, and higher patient acceptability (53, 54). In a retrospective comparative study, Bigatti et al in 2014 reported no significative difference in terms of duration of resection between the Integrated Bigatti Shaver (IBS) (Karl Storz SE & Co KG Tuttlingen Germany) versus conventional bipolar resectoscope. The IBS was able to approach all kind of submucosal myomas in a single-step procedure and in a very precise and easy way (55). It appears that diode laser through “see-and-treat” hysteroscopy and shavers are safe and effective during a myomectomy. However, more studies with larger sample size are needed. The gold standard remains double-flow bipolar resectoscope to remove submucous myomas. The best treatment choice depends on the patient’s personal objectives and the efficacy of each therapeutic option.
Recommendation 14: The panel recommends the use of resectoscopes, lasers or shavers to remove submucous myomas (strong recommendation, high quality evidence).
The panel recommends thorough evaluation of the state of the myoma and proper allocation before selecting the type of surgery (strong recommendation, high quality evidence).
The panel recommends using resectoscopes, shavers or lasers for group I myomas – refer to Table 5 (strong recommendation, high quality evidence).
The panel recommends using two-step hysteroscopy in group II myomas – refer to Table 5 (strong recommendation, high quality evidence).
The panel recommends not using hysteroscopy for group III myomas – refer to Table 5 (strong recommendation, high quality evidence).
Should post-op treatment with IUCD, antiadhesion, balloon catheter be used in prevention and management of Asherman syndrome? Intrauterine adhesions (IUA) are conditions where intracavitary granulation tissue is formed because of injury to the basalis layer of the endometrium, creating fibrous tissue bridges inside the uterine cavity. In the most severe cases, the uterine cavity may be completely obliterated, without any evidence of a healthy endometrium. Hysteroscopy offers direct visualization of the uterine cavity and allows the lysis of adhesions through mechanical or electrosurgical energy.
Primary prevention of intrauterine adhesions: Primary prevention should be considered in the routine clinical practice if intrauterine surgery has been performed 1. minimalizing the damage to the normal tissue by optimizing visibility, precisely cutting and minimizing of the operation time, 2. reducing the thermal effect by electrosurgery and 3. the application of biomaterial and/or barrier agents after procedure, based on uncertainty of ideal treatment for the established IUA and unpredictable outcomes after IUA treatment. Preoperative preparation by medication, namely (gonadotropin-releasing hormone agonist (GnRH agonist), selective progesterone receptor modulator (SPRM), and others are often used before hysteroscopic tissue removal, including hysteroscopic myomectomy. However, Taskin and colleagues found that pretreatment-induced hypoestrogenism did not affect the frequency and severity of IUA formation (57). Strategies of primary prevention of intrauterine adhesions contain at least three parts. The first step for reducing the risk of IUA formation is a delicate surgical technique and the application of minimally traumatic instruments. The second step is careful choice of an energy system for hysteroscopic resection procedure, because it is associated with the development of IUA. As for the third recommended step, it involves the application of barrier methods for primary prevention of IUA. Barrier methods including physical barriers, mechanical barriers, or combination of both.
Recommendations for Primary Prevention of Intrauterine Adhesions: The panel suggests that the use of primary prevention, IUA depending on the procedure being conducted (conditional recommendation, moderate quality evidence).
Management of Intrauterine Adhesions: Hysteroscopic lysis of adhesions is considered the gold standard in management of IUAs. It enables accurate diagnosis and classification as well as instant dissection. Techniques of blunt and sharp dissection under direct visualization for release and excision of adhesions may be performed using cold scissors, electrosurgical instruments or Nd-YAG laser. The more lateral the adhesions and the greater their density, the more difficult the dissection and the greater the risk of complications such as uterine perforation and bleeding.
Recommendations for management of IUAs: The panel recommends the use of hysteroscopic lysis of adhesions by direct visualization and a tool for adhesiolysis for symptomatic IUAs for experienced hysteroscopists (strong recommendation, high quality evidence).
Secondary Prevention of Intrauterine Adhesions: A challenging issue that arises when treating patients with moderate or severe IUA is the recurrence of the adhesions, which is typically estimated as occurring in 3%–25% of cases but has been reported in up to 60%. A systematic review and network meta-analysis of randomized controlled trials of intrauterine interventions options for preventing recurrence after hysteroscopic adhesiolysis was conducted by Ruonan Tang et al 2024. Data from 21 randomized controlled trials involving 2406 patients were synthesized, including interventions with balloon, amnion, platelet-rich plasma (PRP), intrauterine device (IUD), hyaluronic acid (HA), platelet-rich fibrin (PRF), and granulocyte colony- stimulating factor (G-CSF). The top 5 interventions for change in AFS scores were: PRP + Balloon (MD = 5.44; 95% CI, 2.63-8.25), Amnion + Balloon (MD = 5.08; 95% CI, 2.71-7.44), IUD + Balloon (MD = 4.89; 95% CI, 2.49-7.30), HA + Balloon (MD = 3.80; 95% CI, 1.78-5.82), and G-CSF + Balloon (MD = 3.84; 95% CI, 1.05-6.63). There were no statistically significant
differences between interventions in the recurrence rate of moderate-to-severe uterine adhesions and the clinical pregnancy rate (58).
Recommendations for secondary prevention of IUAs: The panel suggests the use of an IUD, stent or catheter to reduce the rate of postoperative adhesion reformation (conditional recommendation, moderate quality evidence).
The panel suggests the use of postoperative hormone treatment using estrogen, with or without progestin following hysteroscopic-directed adhesiolysis (conditional recommendation, moderate quality evidence).
The panel recommends follow-up assessment of the uterine cavity after treatment of IUAs, preferably with hysteroscopy after two to three menstrual cycles following surgery (strong recommendation, high quality evidence).
Could a resectoscope vs no resectoscope be used in treatment of isthmocele or niche? A Cesarean scar defect (CSD) or niche was first described by Poidevin using hysterosalpingography (HSG) in 1961 as a typical small wedge-shaped morphological defect (59). Since then, different studies have described the characteristics of a niche using various imaging techniques, including transabdominal and transvaginal sonography (TVS). In 2019, the European Niche Taskforce published a consensus definition of the niche in Jordans et al. as; “an indentation of the uterine myometrium at the site of the SC scar with a depth of at least 3 mm” and classified the niche into: simple, simple with a branch and complex (59). A multicenter RCT assessed the effectiveness of a hysteroscopic niche resection versus no treatment in women with postmenstrual spotting and a uterine Cesarean scar defect. A total of eleven hospitals collaborating in a consortium for women’s health research in the Netherlands. 103 women reporting postmenstrual spotting after a Cesarean section who had a niche with a residual myometrium of ≥3 mm, were randomly allocated to hysteroscopic niche resection or expectant management for six months. At follow-up, the median number of days of postmenstrual spotting was four days (interquartile range, IQR 2-7 days) in the intervention group and seven days (IQR 3-10 days) in the control group (P = 0.04) compared to eight days at baseline in both groups. Patients in the intervention group also reported a significant decrease in spotting related discomfort (60).
Recommendation 15: The panel recommends hysteroscopic niche resection for women with a cesarean scar defect (CSD) with a residual myometrium of ≥3 mm and related symptoms (strong recommendation, moderate quality evidence).
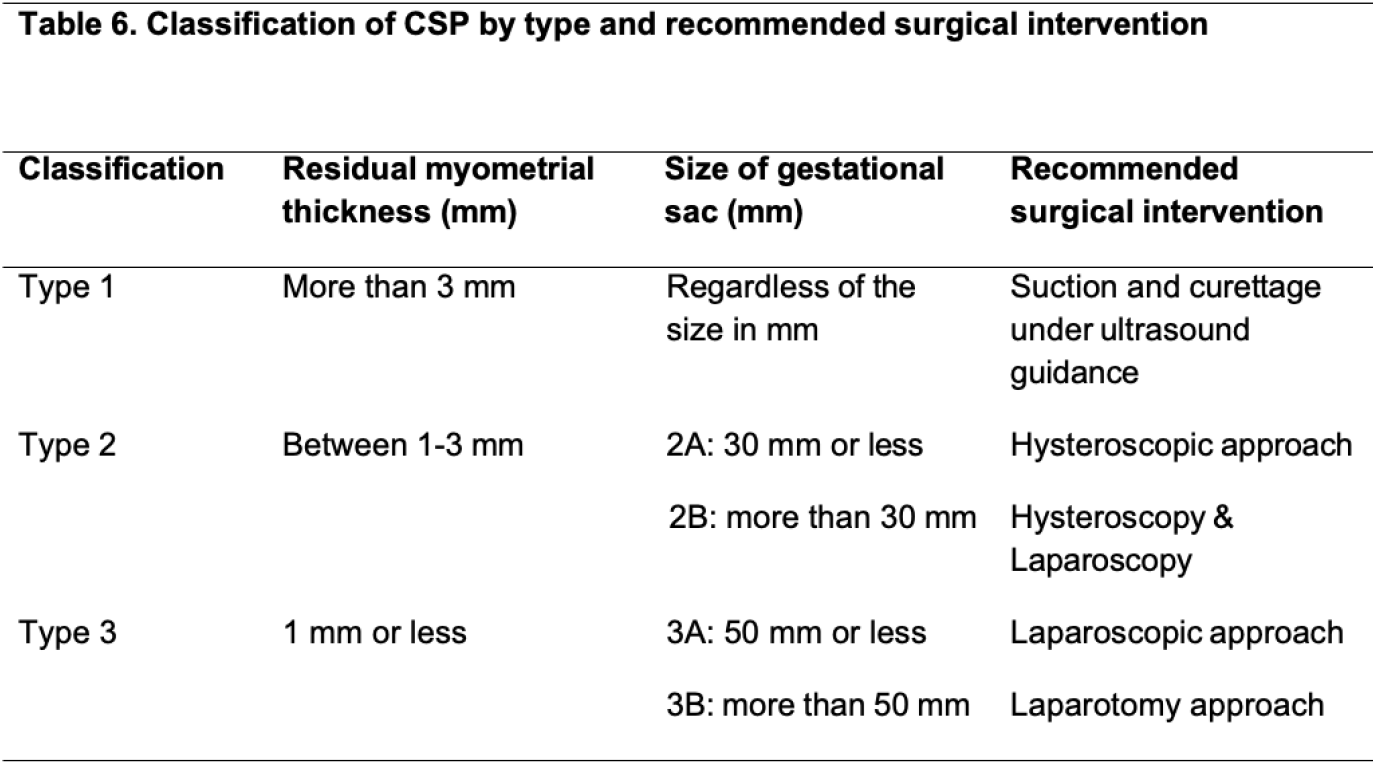
Should resectoscope, laser, or tissue collection system be used in Cesarean scar pregnancy (CSP)? CSP is an early and abnormal implantation of pregnancy on Cesarean section scar. The incidence of CSP has been increased worldwide due to the high rates of Cesarean sections. Without a proper management plan for CSP, risk of maternal morbidity and mortality are high from severe intraabdominal bleeding, uterine rupture, morbidly adherent placenta (MAP), hysterectomy and even death (61). The optimal diagnostic method for CSP is pelvic ultrasound (62), however, in some clinical situations magnetic resonance imaging (MRI) of the pelvis is mandatory for clinical acuity. A new classification of CSP has been published by Jordans et al (Table 6) (63). Vial et al, reported the two types of CSP as exogenic and endogenic based on pelvic ultrasound result (6). While, Ban et al, reported CSP classification into three types (I, II a, III) and depending on some variable items, i.e. anterior myometrial thickness (AMT), gestational sac diameter, to achieve a good clinical outcome. A systematic review assessing hysteroscopic treatment of CSP included four cohort studies where hysteroscopy was used among five groups totaling 329 patients. The studies used uterine curettage under hysteroscopic guidance in three treatment groups and operative hysteroscopy via resection in the other two. The efficacy of the treatment ranged among studies from 64.9% to 96.1%. The complication rates were low, with hemorrhage or excessive vaginal bleeding postoperatively reported in five out of 301 cases (1.66%) and hysterectomy in just one patient (0.33%). The review also reported an acceptable mean operative time, intraoperative blood loss and days of hospital stay (64).
Recommendation 16: The panel suggests the use of hysteroscopy in type I and type IIa CSP (conditional recommendation, low quality evidence). Refer to table on Delphi.
What is the role of hysteroscopy in uterine-sparing treatments for AUB -Adenomyosis? Adenomyosis is a common chronic disease in women of reproductive age, characterized by the presence of ectopic endometrial tissue within the myometrium. The development of ultrasonography and magnetic resonance imaging has improved the pre-operative diagnosis of adenomyosis. Hysteroscopy has been shown to be effective in identifying superficial adenomyosis. However, its role in the surgical management of adenomyosis requires further confirmation through additional studies (65). The management of adenomyosis has evolved with the introduction of hysteroscopy, offering a less invasive alternative to traditional surgical interventions. Endometrial ablation has been explored as a treatment option for adenomyosis in several studies, however the literature is lacking. In cases where the diagnosis of adenomyosis is uncertain, hysteroscopy may serve as a helpful adjunct tool. Changes in the endometrium, such as hypervascularization, can be visualized during hysteroscopy, aiding in the diagnosis of adenomyosis. Treatment with endometrial ablation is limited, although it might assist in reducing excessive bleeding (65).
Recommendation 17: The panel suggests the use of endometrial ablation for the treatment of adenomyosis (conditional recommendation, low quality evidence).
Discussion
Hysteroscopy is the new gold standard in the future because of its ability to visualize directly the endometrium and perform directed biopsies as indicated. As office-based hysteroscopy becomes more practical and widespread, the technique may become more cost effective. An evaluation plan using transvaginal sonography as the initial screening evaluation followed by endometrial biopsy or, more likely, hysteroscopy is likely to become the standard of care (66). Hysteroscopy as a diagnostic tool permits direct visualization of the cervical canal and uterine cavity, enabling observation of intrauterine abnormalities. An accurate diagnosis may result in surgical or medical treatment directed at the specific pathology and may avoid the need for major surgery. Since Gimpelson and Rappold reported that hysteroscopy combined with guided biopsy was more accurate than dilatation and curettage (67), hysteroscopy is considered an accurate ‘gold standard’ in uterine cavity evaluation. Despite the lack of adequate information about the diagnostic accuracy, it is used in many studies with and without endometrial sampling as a reference standard (25, 68-70). The recommendations in this guideline, supported by current literature, emphasize the importance of patient-centered care, technological advancements, and evidence-based practices. With proper training and adherence to established protocols, hysteroscopy remains a cornerstone in the diagnostic and therapeutic armamentarium of gynecology.
Conclusion
The recommendations made in this guideline should not be viewed as dictates and it should be clear that even the strongest of recommendations may not apply across all patients and conditions. Overall, this evidence-based guideline was set forth by a group of experts in the field with specialties of relevance to hysteroscopy. These recommendations were drafted with the highest medical standards in mind and supported by the Saudi Ministry of Health. Our review of the literature provided us with some answers to our PICO questions and we have summarized our findings and expert opinions in the recommendations included here- in. The literature however is insufficient in some cases and making an informed decision based on the available data was challenging. The literature is also lacking in the region and in Saudi Arabia in particular, with regards to use of hysteroscopic procedures for diagnostic and operative purposes and patient satisfaction.