Authors / metadata
DOI: 10.36205/trocar6.vid25009
Abstract
Deep infiltrating endometriosis extends beyond the peritoneum, often affecting the rectosigmoid colon, requiring complex surgical management. The previously published “En Bloc Hysterectomy Technique” describes the simultaneous resection of the uterus and affected bowel through intestinal resection (1). Building upon this approach, a structured 10- step technique to enhance precision, reproducibility, and surgical efficiency has been developed. This standardized method optimizes dissection, improves tissue preservation, and reduces intraoperative time, risk of rectal fistulas, and postoperative complications (2). The “En Bloc Hysterectomy in 10 Steps” provides a systematic, reproducible strategy for managing intestinally infiltrative endometriosis requiring hysterectomy, offering improved safety and surgical outcomes.
Learning objective
To provide a structured, reproducible 10-step approach for performing En Bloc Hysterectomy in cases of deep infiltrating endometriosis with intestinal involvement, aiming to enhance surgical precision, optimize tissue preservation, and reduce intraoperative time and complications.
Introduction
DE is defined by the presence of endometrial-like tissue extending beyond the peritoneal surface, affecting not only reproductive organs but also adjacent structures, particularly the intestinal tract. Among extragenital locations, the rectosigmoid colon is one of the most frequently involved sites, with an estimated 5% to 37% of patients with DE presenting intestinal infiltration. This condition not only leads to severe pelvic pain but also contributes to gastrointestinal symptoms such as dyschezia, constipation, rectal bleeding, and, in severe cases, partial bowel obstruction. Surgical management of colorectal endometriosis poses significant challenges, with reported complication rates of 10–22% when bowel resection is required (3). The choice of surgical approach depends on multiple factors, including lesion size, depth of invasion, and the extent of bowel wall involvement (1,4). Treatment strategies range from conservative techniques, such as rectal shaving or discoid resection, to radical approaches, including segmental resection when full-thickness infiltration is present (3). A standardized and reproducible surgical technique is crucial to improving outcomes and minimizing complications in these high-risk procedures (1,4). The En Bloc Hysterectomy in 10 Steps was developed as a systematic approach to ensure surgical precision, enhance safety, and optimize patient recovery in cases of intestinally infiltrative endometriosis requiring hysterectomy.
Methods
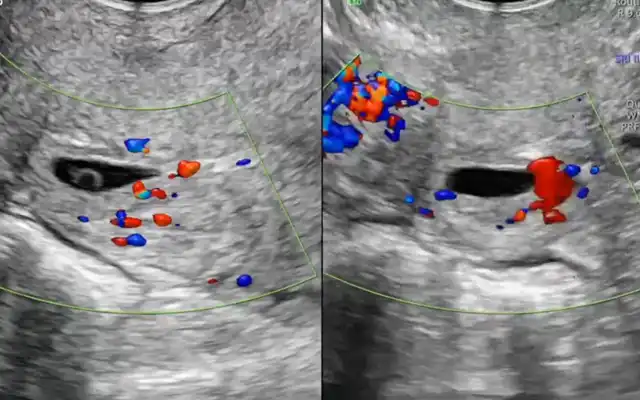
The case of a 46-year-old patient with a history of dysmenorrhea, chronic pelvic pain, dyschezia, severe dyspareunia, and chronic constipation is presented. A mandatory preoperative endometriosis mapping was performed, including pelvic MRI, which revealed the following findings: Diffuse adenomyosis A 4 cm endometriotic plaque emerging from the uterosacral torus, adhering the rectum at 12 cm from the anal verge, involving 40% of its circumference. Retracted tubo-ovarian complexes with endometriomas adherent to the bowel (#E) v2021: P0, O3/3, T3/3, A2, B1/1, C3, FA The procedure performed was a minimally invasive laparoscopic en bloc hysterectomy with segmental bowel resection. For didactic purposes, the video was structured into 10 standardized surgical steps to allow a clearer understanding and reproducibility of the technique, aiming to improve surgical efficiency and safety:
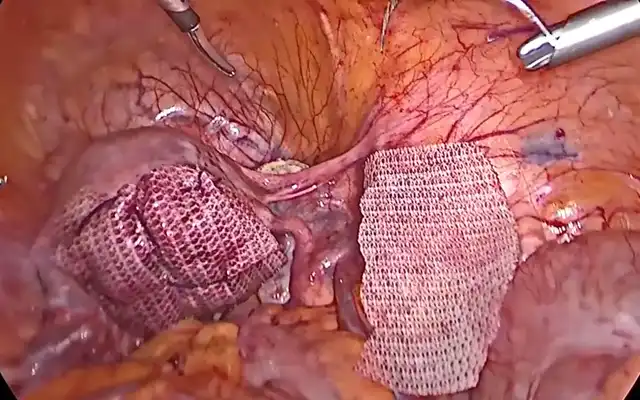
- Dissection of the three hermetic pelvic zones
- Uterine artery coagulation in Latzko’s space
- Dissection of the vesicouterine space
- Dissection of the left Latzko’s and Okabayashi’s spaces
- Ligation of the sigmoid artery with Hem-olok clips
- Anterior colpotomy to initiate en bloc hysterectomy
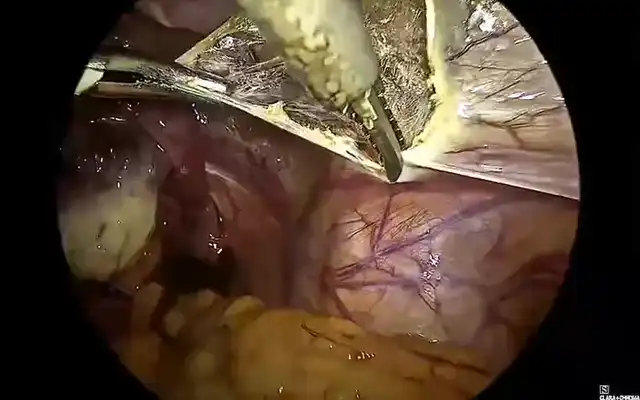
- Dissection of the rectovaginal space
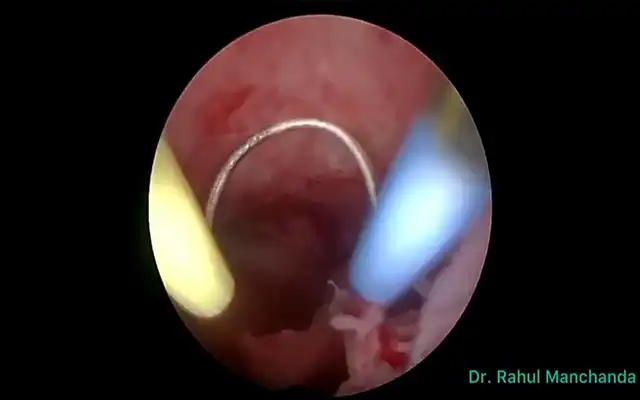
- Distal bowel resection with linear stapler
- Placement of the anvil in the proximal end through mini-laparotomy
- Closure of the vaginal cuff
Main outcome
The total operative time was 160 minutes. Intraoperative monitoring was performed using procalcitonin and C-reactive protein levels as markers for potential anastomotic leakage; all remained within normal ranges. The patient was discharged on postoperative day two, tolerating an anti-inflammatory diet without complications (6,7).
Results
One month after surgery, the patient showed significant improvement in symptoms, with the Visual Analog Scale (VAS) for pelvic pain decreasing from 8/10 to 3/10. Histopathology revealed diffuse adenomyosis and chronic inflammation with edema in the colonic muscularis, accompanied by a fibromuscular nodule consistent with deep endometriosis.
Discussion
DE is a complex and progressive disease characterized by the infiltration of endometriotic tissue beyond the peritoneum, often affecting adjacent structures such as the intestinal tract. Surgical management becomes particularly challenging when the disease involves the rectosigmoid colon, necessitating advanced surgical techniques to achieve complete disease excision while minimizing complications (3-5). The well-established and previously published “EBH” describes the simultaneous resection of the uterus and the affected portion of the rectosigmoid colon through a segmental resection (1). Previous studies have highlighted the benefits of this approach in achieving radical disease excision while minimizing complications (8,9). Building upon this knowledge, a structured 10-step approach to further standardize the procedure, ensuring a reproducible and applicable methodology for surgeons managing complex cases of DE with colorectal involvement has been developed. This systematization aims to make the technique more accessible and easier to implement for specialists worldwide.
The “EBH in 10 Steps” offers numerous surgical advantages, primarily by optimizing dissection, improving anatomical visualization, and reducing intraoperative risks (1,10). By adhering to a structured surgical roadmap, this technique enhances precision, facilitates clear tissue planes, and minimizes the likelihood of inadvertent injury to adjacent structures. One of the key advantages of this approach is the reduction in operative time. By systematically addressing affected tissues in a controlled manner, the procedure eliminates unnecessary steps, reduces intraoperative decision- making complexity, and expedites the excision process. This efficiency is particularly beneficial in long, complex surgeries where prolonged operative times are associated with increased risks of blood loss, infection, and postoperative complications (1,8,9).
Additionally, the improved anatomical visualization of the rectovaginal space is a critical benefit of this technique. Through strategic and systematic dissection, the rectovaginal septum can be adequately mobilized, allowing for a precise separation of the rectum from the vaginal plane. This ensures better control of the affected area and significantly decreases the risk of rectal perforation during dissection, which remains one of the most concerning complications in colorectal endometriosis surgery (1,4,9).
The reduction of intestinal complications, particularly the risk of rectal fistulas, is another significant advantage of this technique. By enabling meticulous dissection before addressing the most deeply infiltrated structures, the “EBH” technique ensures that healthy tissue planes are preserved, minimizing trauma to the bowel. This targeted approach decreases the need for extensive bowel resections, promoting a more conservative intestinal-sparing strategy when applicable (1). Furthermore, the “EBH” approach enhances nerve preservation, particularly in cases with extensive parametrium and rectosigmoid involvement. By clearly delineating and mobilizing key anatomical structures, such as the hypogastric nerves and ureters, the risk of long-term complications, including bladder dysfunction and neuropathic pain, is significantly minimized.
Regarding anti-inflammatory diet, recent evidence suggests that chronic inflammation plays a central role in the pathophysiology of endometriosis. Anti- inflammatory dietary interventions have been shown to modulate systemic and local immune responses by reducing the release of pro-inflammatory cytokines and oxidative stress markers. Therefore, an anti-inflammatory diet is recommended as part of the preoperative preparation in selected patients to potentially improve surgical outcomes by reducing tissue edema and pelvic inflammatory activity (6-7).
Video
Conclusion
The “EBH in 10 Steps” represents an evolution in the surgical management of DIE with colorectal involvement, offering a highly standardized, reproducible, and efficient technique. While the benefits of “EBH” have been previously documented, this article systematizes the procedure into a 10-step approach, ensuring greater accessibility and reproducibility for surgeons. The structured nature of this approach improves surgical precision, optimizes patient safety, and minimizes complications, making it a valuable tool for surgeons specializing in advanced endometriosis surgery. As experience with this technique grows, further research and multicentric validation studies will be essential to evaluate its role as the gold standard for complex cases of DIE with intestinal involvement.