Authors / metadata
DOI: 10.36205/trocar6.2025011
Abstract
Endometriosis is a chronic inflammatory disease affecting approximately 10% of reproductiveaged women worldwide. It is characterized by the presence of endometrial-like tissue outside the uterus, often infiltrating pelvic structures such as the bowel, bladder, and ureters. Deep infiltrating endometriosis (DE) presents a particular surgical challenge due to extensive fibrosis, anatomical distortion, and the risk of injury to adjacent structures. In recent years, fluorescence-guided surgery (FGS) using indocyanine green (ICG) has emerged as a valuable tool to enhance surgical safety and precision in minimally invasive procedures.
ICG is a near-infrared fluorophore that, when administered intravenously or locally, facilitates real-time visualization of vascular and anatomical structures. Its intraoperative applications include identifying critical structures (biliary tree, ureters, parathyroids, and thoracic duct), evaluating tissue perfusion (colorectal anastomosis, ischemic bowel, and flap viability), and guiding lymphatic mapping in oncologic surgery. In endometriosis surgery, ICG has demonstrated effectiveness in ureteral identification, ensuring preservation of ureteral integrity during resection of deeply infiltrating lesions. Due to its hepatic metabolism, ICG must be administered directly into the ureters via cystoscopic catheterization to achieve fluorescence, providing continuous intraoperative guidance during complex pelvic dissections. Furthermore, fluorescence angiography with ICG plays a crucial role in colorectal surgery, particularly in the assessment of anastomotic perfusion. Anastomotic leakage remains a major concern, occurring in up to 19% of colorectal procedures. Studies have shown that ICG fluorescence significantly reduces anastomotic complications by allowing real-time perfusion assessment. The European Association for Endoscopic Surgery (EAES) has endorsed fluorescence-guided perfusion evaluation as a method to improve surgical outcomes and patient safety.
Fluorescence-guided surgery in endometriosis represents a promising advancement in minimally invasive gynecologic and colorectal procedures. The use of ICG enables precise identification of ureters, improves anastomotic safety, and facilitates complex pelvic dissections. Standardizing ICG dosing, administration protocols, and fluorescence interpretation criteria will be essential to optimizing its clinical utility in endometriosis surgery.
Introduction
Endometriosis is a chronic inflammatory disease defined as the presence of endometrial glands and stroma outside the uterine cavity, primarily in the pelvis but potentially extending to extrapelvic locations. It is estimated that approximately 10% of reproductive-aged women are affected by endometriosis worldwide (1). Surgical management remains the standard treatment for severe cases, particularly in deep infiltrating endometriosis (DE) with colorectal involvement (2). However, these surgeries are challenging due to anatomical distortion caused by fibrosis and inflammation. The use of fluorescence-guided techniques has gained popularity in minimally invasive surgery, offering enhanced visualization of anatomical structures and improving surgical safety (3).
Discussion
Indocyanine green (ICG) is a cyanine-based dye that, when injected intravenously, allows near-infrared fluorescence imaging of various tissues and structures (4). Its intraoperative applications range from identifying anatomical landmarks (biliary tract, ureters, parathyroids, thoracic duct) to assessing tissue perfusion (colorectal anastomoses, gastric and bariatric surgery, ischemic bowel, flap viability) and mapping sentinel lymph nodes (3). In endometriosis surgery, ICG has been reported as a safe and effective tool for delineating anatomy and assessing disease extent (5).
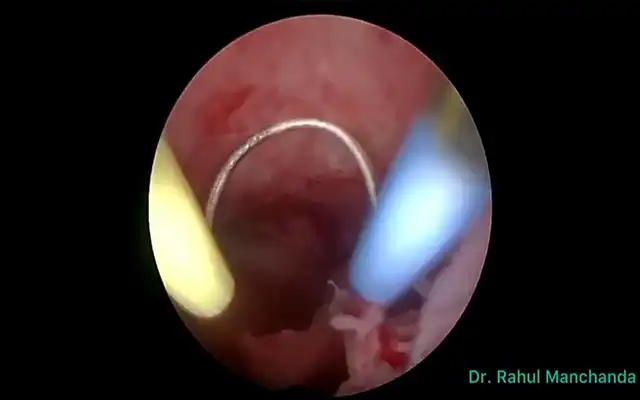
One of the major challenges in colorectal and gynecological surgeries is ureteral injury, particularly in cases of DE. Ureteral damage has been reported in 0.15% to 0.66% of colorectal surgeries, with pelvic reconstructive surgeries presenting an even higher risk (up to 11%) (6,7). ICG can be instilled into the ureter through a catheter during cystoscopy, enabling real-time visualization and preventing iatrogenic injury. This method is necessary since intravenously administered ICG is hepatically cleared and does not reach the ureters (3).
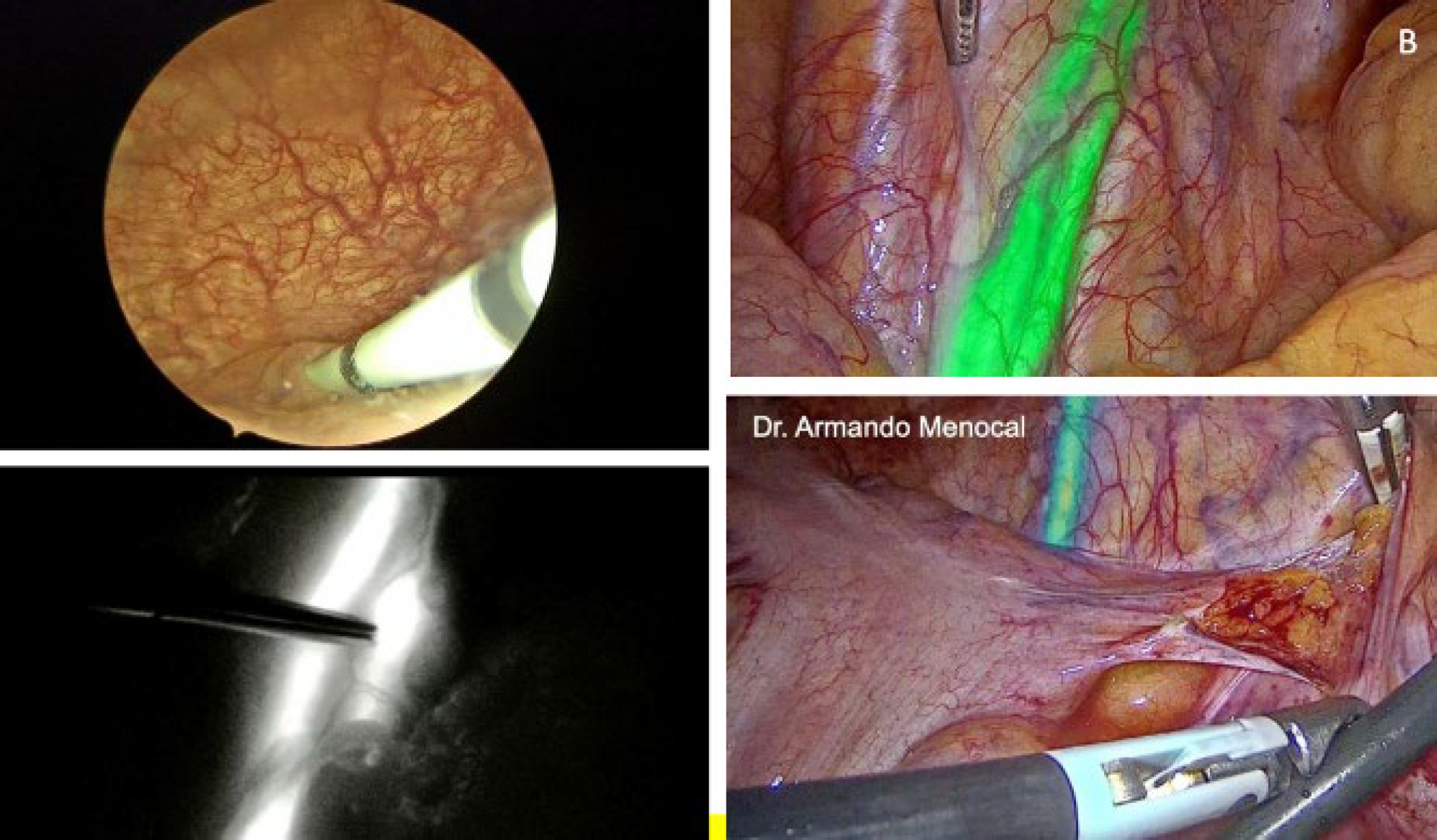
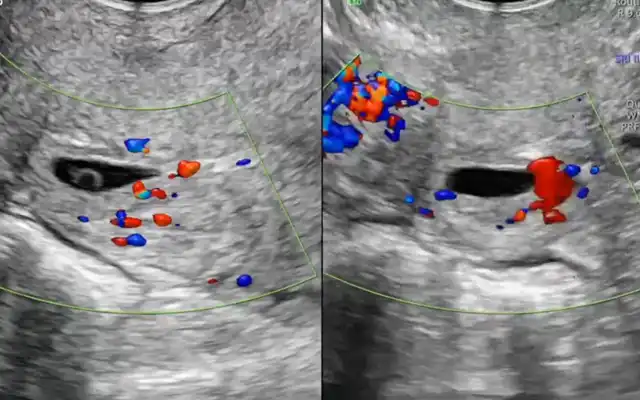
Figure 1: (upper left) Catheterizing the ureter meatuses via cystoscopy to administer bilateral indocyanine green, at a dose of 2.5 mg/ 10 ml per ureter in sterile solution. Fig 1B) (Upper right) Identification of the ureter using indocyanine green, in this case with a duplicated ureteral system. Fig 1C) The same duplicated ureteral system under monochromatic visualization. Fig 1D) ureteral identification using indocyanine green fluorescence with intensity mapping mode.
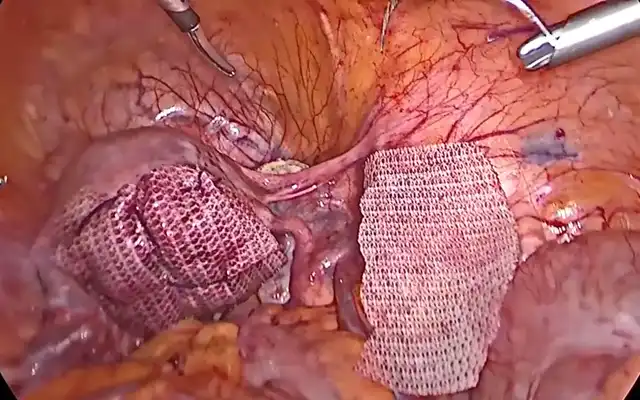
Colorectal anastomoses pose technical challenges, with anastomotic leakage occurring in up to 19% of cases (8). ICG fluorescence angiography has emerged as a valuable tool for assessing tissue perfusion intraoperatively, significantly reducing the risk of anastomotic failure (9). The European Association for Endoscopic Surgery (EAES) endorses ICG use in colorectal surgery to enhance safety and surgical outcomes (10).
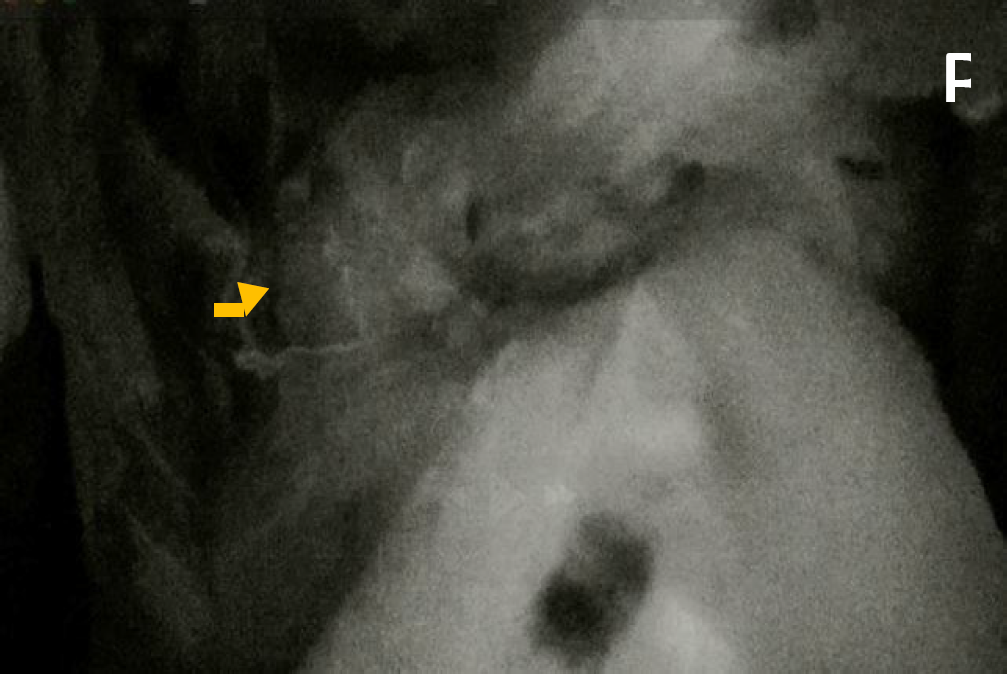
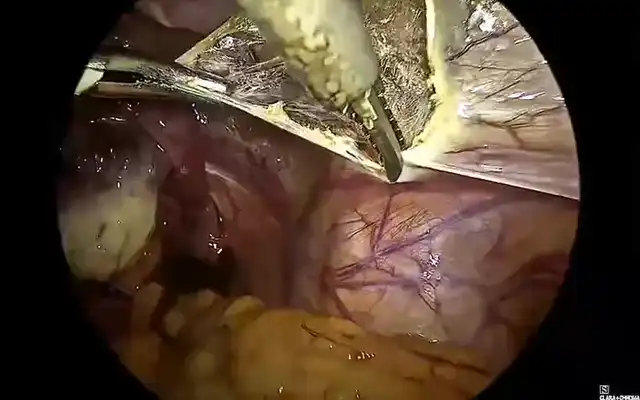
Figure 2 A) The white arrows indicate the staple line created with a circular stapler following a segmental resection. 2 B) Perfusion of the resection site is evaluated using indocyanine green (ICG) fluorescence imaging. Assessment could be performed both prior to and following (yellow arrow) the anastomosis to ensure adequate tissue perfusion. decision making to optimize anastomotic integrity and reduce the risk of ischemic complications.
Different methods exist for evaluating blood flow to anastomotic sites, but traditional visual assessment (serosal discoloration, pulsatile bleeding at the cut edge, or marginal artery flow) lacks precision in detecting micro perfusion deficits. Fluorescence angiography allows real-time perfusion assessment, with optimal evaluation occurring 1.5 to 3.5 minutes after intravenous administration of 2.5 mg of ICG at a 5 cm camera distance from the colon (11,12). Figure 2B. Also, for identifying and mobilizing the rectum with a rectal manipulator.
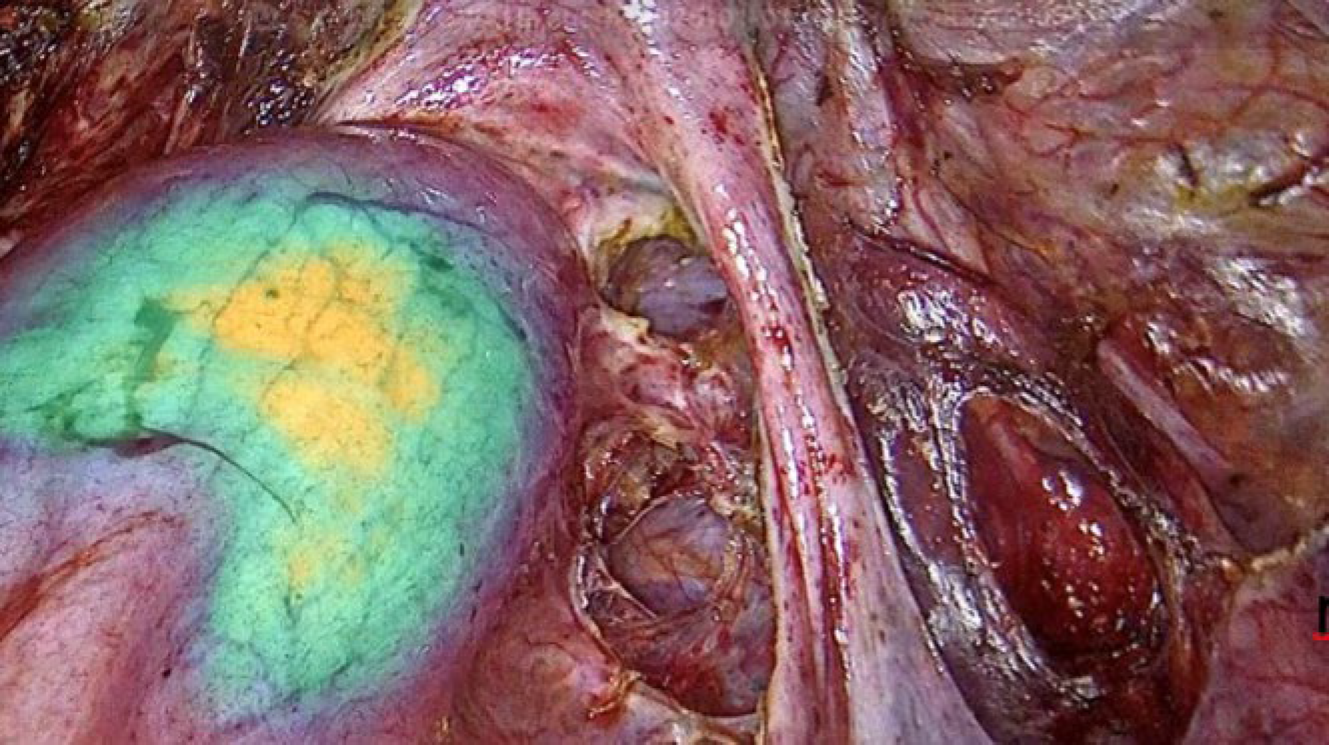
Figure 3: An intrarectal manipulator equipped whit near-infrared imaging was employed to enhance delineation of the rectal wall. In specific cases, this technique facilitated the identification and precise localization of endometriotic lesions infiltrating the rectal wall.
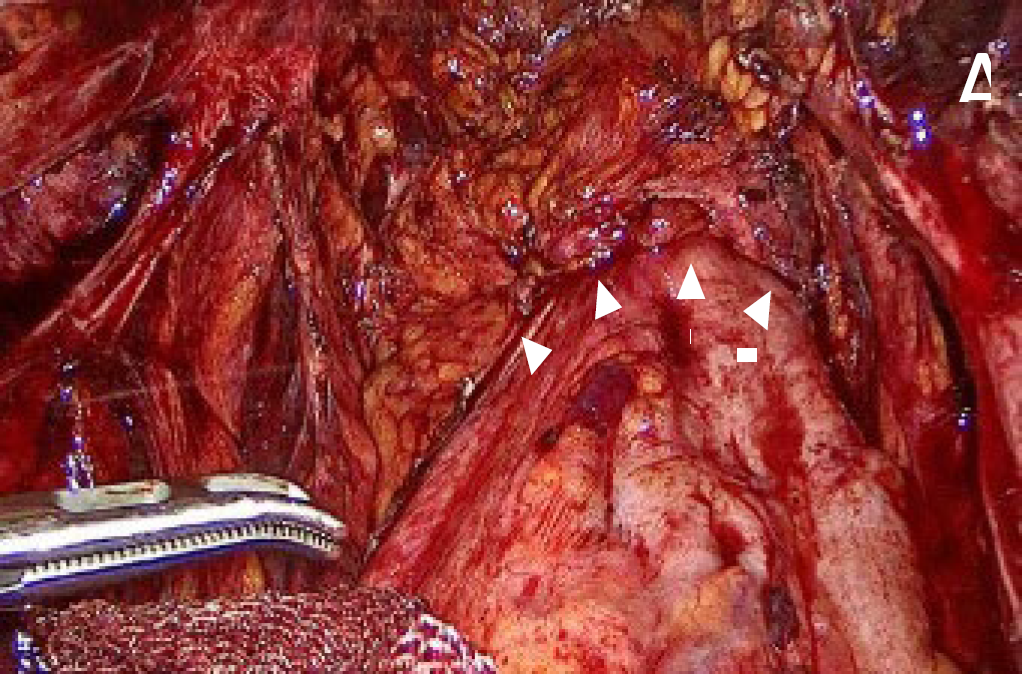
In deep endometriosis surgery, complex pelvic surgery and oncology surgery, the use of ICG allowed the highlighting of the hypogastric nerve and inferior hypogastric plexus, that facilitated the visualization and preservation in the surgery to avoid nerve injury (13)
Conclusion
In conclusion, fluorescence-guided surgery using ICG provides a reliable intraoperative anastomosis outcome.