Authors / metadata
DOI: 10.36205/trocar6.2025013
Abstract
This video presents a standardized laparoscopic approach for en bloc triple-compartment excision in a patient with deep endometriosis (DE) involving the bladder, uterus, and rectosigmoid colon. The disease was staged using the #ENZIAN 2021 classified as P0, O2/0, T3/1, A2, B1/1, C3, FA, FB, F1. A nerve-sparing dissection strategy and reverse anatomical technique were utilized to enhance surgical safety and preserve the pelvic autonomic nerves. Intraoperative fluorescence with indocyanine green (ICG) was employed to identify the ureters and confirm adequate vascular perfusion prior to bowel transection. The procedure included full-thickness excision of the bladder nodule with a two-layer closure, total laparoscopic hysterectomy with bilateral ureterolysis, and segmental resection of the rectosigmoid colon, followed by a side-to-side intracorporeal anastomosis using a linear stapler. The specimen was extracted via transvaginal Natural Orifice Specimen Extraction (NOSE). This case demonstrates the feasibility of a minimally invasive, triple-block approach for advanced DE using a compartmental and function-preserving surgical philosophy. The integration of nerve-sparing principles, reverse dissection, and fluorescence-guided surgery contributes to anatomical precision and operative safety. The #ENZIAN classification served as a valuable framework for mapping, staging, and guiding a multidisciplinary surgical strategy in complex pelvic disease.
Learning objective
To implement standardized laparoscopic strategies for triple-compartment excision in deep endometriosis (DE), with emphasis on nerve preservation, identification of neurovascular structures, and complete bloc excision of lesions in the bladder, uterus, and rectosigmoid colon.
Introduction
Deep endometriosis (DE) is a chronic, inflammatory condition defined by the presence of endometrial-like glands and stroma infiltrating more than 5 mm into peritoneal or visceral surfaces. DE frequently affects multiple compartments of the pelvis—anterior (bladder and ureters), central (uterus, cervix, parametrium), and posterior (rectovaginal septum, uterosacral ligaments, and rectosigmoid colon)— leading to pelvic pain, infertility, and dysfunction of urinary and gastrointestinal organs (1,2).
Among the most complex presentations is multicompartmental DE, which requires advanced laparoscopic skills, anatomical mastery, and often a multidisciplinary approach. Bladder endometriosis accounts for 1–2% of DE cases and is associated with dysuria, urgency, or cyclic hematuria when lesions infiltrate the detrusor (3). Rectosigmoid involvement may lead to dyschezia, altered bowel habits, or even partial obstruction. Concurrent adenomyosis and uterine fibrosis contribute to severe central pelvic pain and can limit surgical dissection planes (4).
Laparoscopic surgical excision remains the gold standard in symptomatic DE, particularly when organ function is compromised or when medical therapy fails. However, aggressive resection near critical structures such as the hypogastric plexus, ureters, and parametrial vessels risks long-term morbidity if nerve-sparing techniques are not employed (5,6). The development of nerve-sparing laparoscopic approaches, together with tools such as fluorescence-guided imaging using indocyanine green (ICG), has enabled safer resection with preservation of bladder and bowel function (7).
The reverse laparoscopic technique, introduced by Kondo et al., emphasizes peripheral to central dissection, improving exposure and minimizing neural damage in fibrotic areas (8). In parallel, the #ENZIAN classification (2021 update) allows structured preoperative planning and intraoperative mapping, staging lesions by location (anterior: A, posterior: B, rectosigmoid: C), extent (1–3), and extragenital involvement (F compartments) (9).
This manuscript presents a case of laparoscopic triple-block en bloc resection involving the bladder, uterus, and rectosigmoid in a patient with severe DIE, demonstrating a standardized, function-preserving approach based on reverse dissection, fluorescence-guided surgery, and nerve-sparing principles.
Patient and Methods
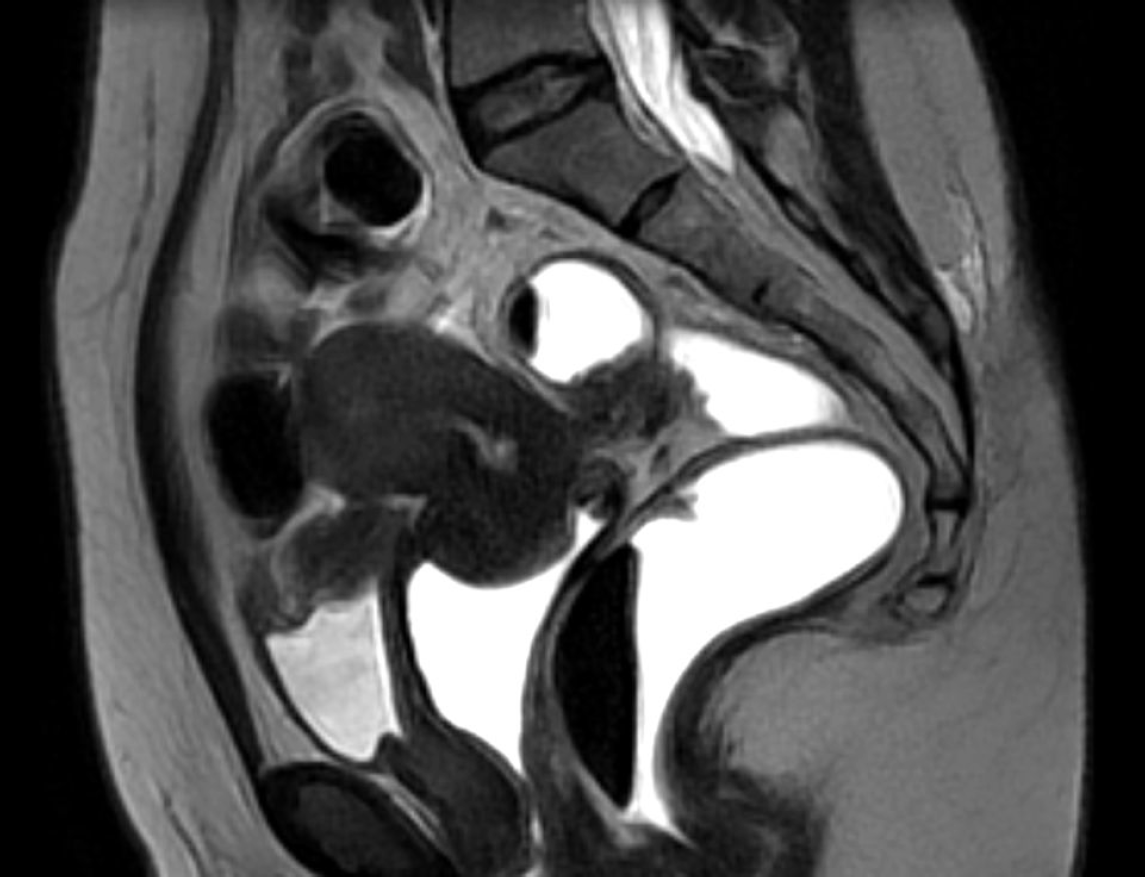
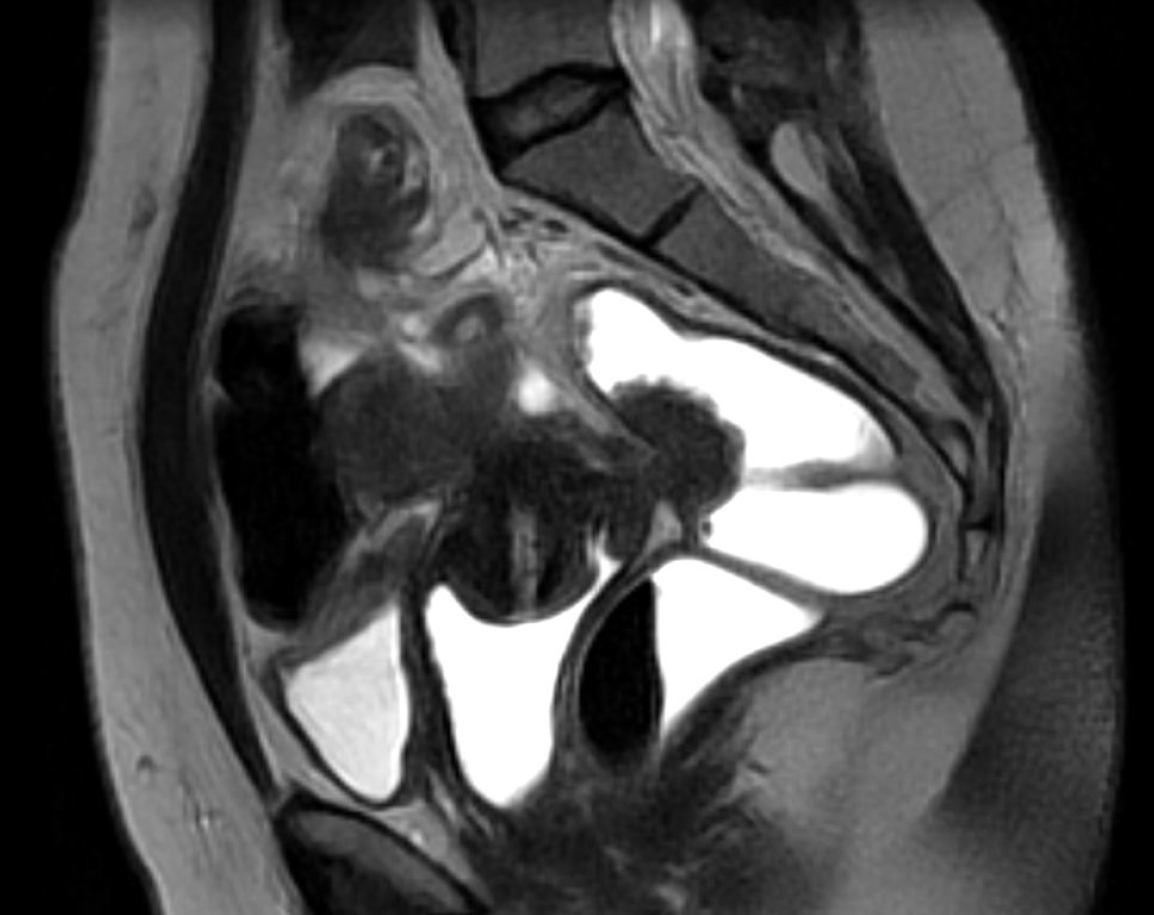
The case of a 41-year-old patient, with a long-standing history of chronic pelvic pain, dysuria, cyclic hematuria, dyschezia, and dyspareunia is presented. Given the multifocal symptoms suggestive of anterior and posterior compartment involvement, an endometriosis mapping protocol was performed using high- resolution pelvic MRI. The imaging revealed left endometrioma, extensive adenomyosis with posterior uterine wall thickening, a full-thickness endometriotic lesion infiltrating the bladder dome and an additional lesion on the posterior bladder wall (Figure 1). A transmural rectosigmoid nodule measuring 3.4 cm in diameter was identified approximately 10–12 cm from the anal verge, with marked serosal retraction and submucosal thickening (Figure 2). #ENZIAN 2021 classification: P0, O2/0, T3/1, A2, B1/1, C3, FA, FB, F1.
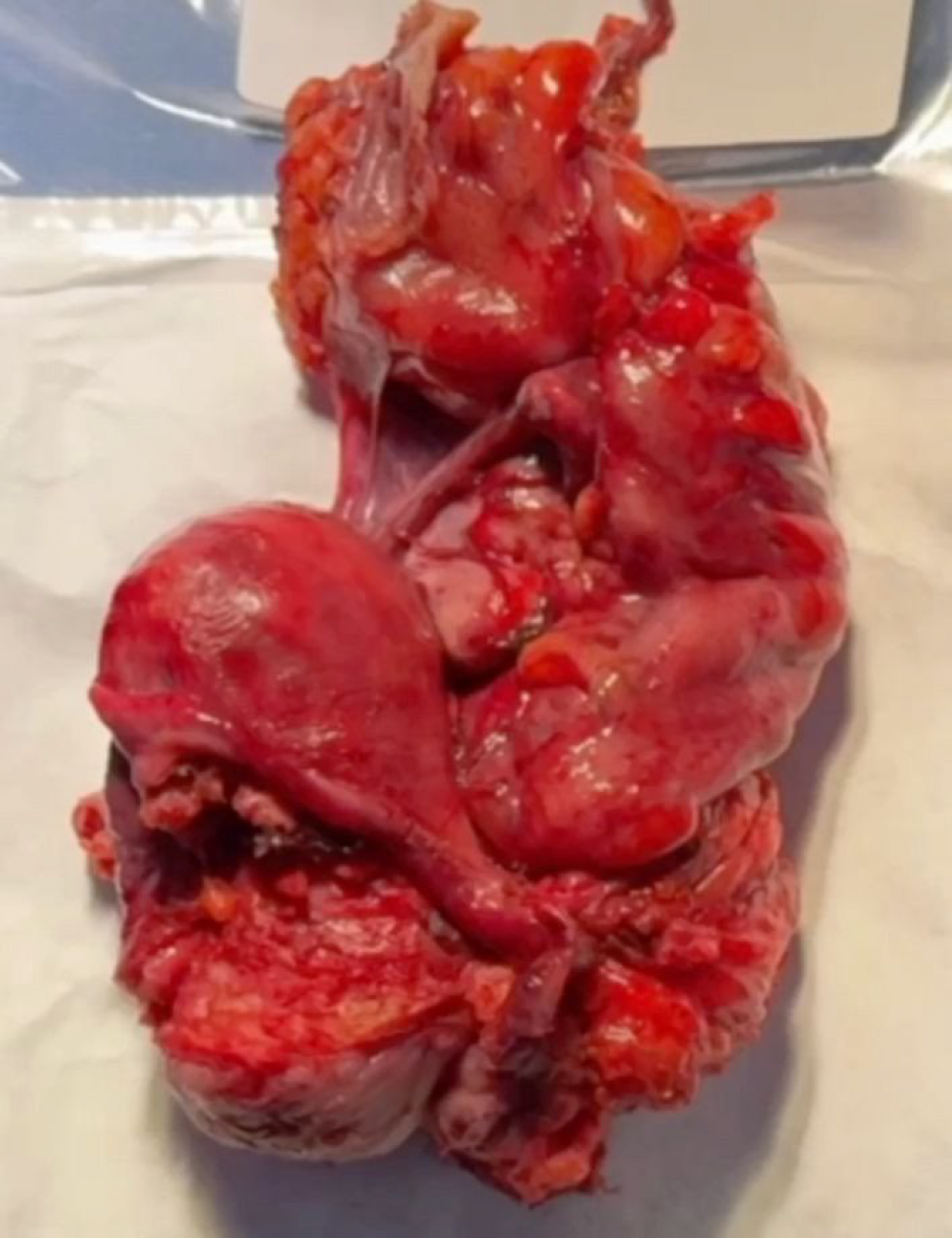
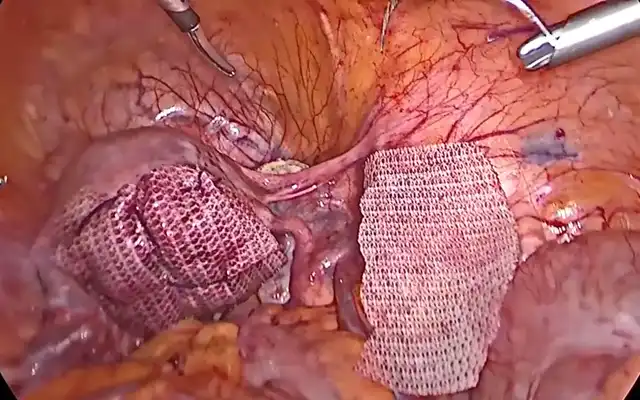
The surgical strategy was planned accordingly. The patient provided informed consent for use of anonymized clinical and surgical data for scientific publication. Surgical excision was performed via a minimally invasive, nerve-sparing laparoscopic approach, and the final specimen—including uterus, bladder wall segment, and intestinal segment—was documented after en bloc extraction, highlighting the topographical relationship of each compartment involved (Figure 3).
Surgical Technique
Under general anesthesia, the patient was placed in the Lloyd-Davies position. Pneumoperitoneum was created via a 10 mm trans umbilical trocar (12 mmHg), and three accessory 5 mm trocars were inserted in both lower quadrants and suprapubic region. A 30° laparoscope was used for panoramic inspection and guidance throughout the procedure.
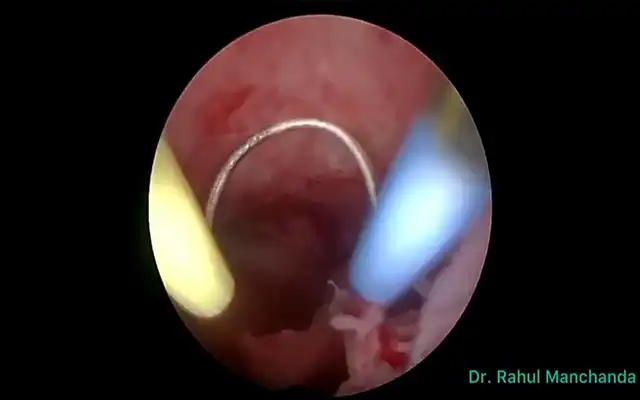
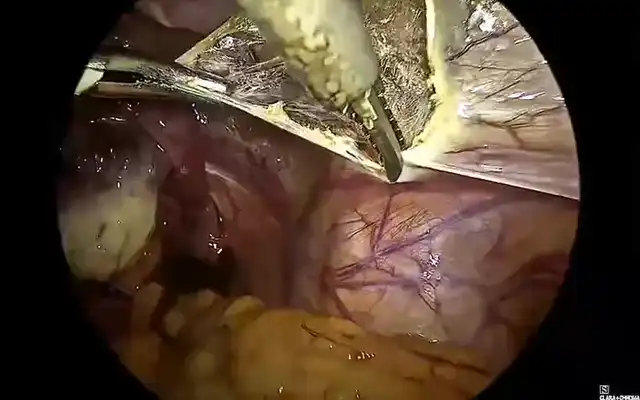
A systematic pelvic peritonectomy was performed from lateral to medial using ultrasonic and bipolar energy, following the reverse laparoscopic technique. Bilateral transection of the round ligaments allowed dissection of the vesicouterine and medial paravesical spaces. The anterior leaf of the broad ligament was opened to expose the Latzko spaces and major vascular structures, including the uterine arteries and superior vesical vessels. Okabayashi space dissection was completed bilaterally to preserve the inferior hypogastric plexus. The parametrial nodule encasing the right ureter was carefully excised, followed by meticulous ureterolysis. A total laparoscopic hysterectomy was performed via anterior and posterior colpotomies. The bladder nodule was excised full-thickness using ultrasonic energy, and the defect was closed in two layers with a 3-0 barbed suture. The rectosigmoid lesion was identified and resected segmentally, with ligation of mesenteric vessels close to the bowel wall to avoid devascularization and neural injury. A side-to-side intracorporeal anastomosis was created using a 60 mm linear stapler. Intestinal perfusion and anastomotic integrity were confirmed with ICG fluorescence and methylene blue leak test.
The entire specimen including uterus, bladder wall, and bowel segment was extracted transvaginally via a posterior colpotomy. The vaginal cuff closure was performed with a 0 Monocryl suture. The procedure followed a structured reverse anatomical dissection, enabling complete compartmental excision with maximal neurovascular preservation.
Intraoperative Findings
Upon entry into the abdominal cavity, extensive pelvic adhesions were noted. The uterus was densely adherent to the anterior rectal wall via the posterior mesorectum. Both ovaries were fixed to the posterior uterine wall. A nodular lesion infiltrating the dome of the bladder was clearly visible. The left ureter was partially encased by a firm, fibrotic parametrial nodule. Fluorescence guidance using Indo-Cyanine Green (ICG) allowed for direct intraoperative nerves sparing. The rectosigmoid junction was significantly thickened and retracted by a transmural endometriotic lesion. No ascites or extra pelvic lesions were observed.
Discussion
The complexity of deep endometriosis surgery lies not only in achieving complete excision but in doing so while preserving neurological, urological, and gastrointestinal function. The presented case demonstrates the feasibility of managing a triple compartment DE, through a single minimally invasive laparoscopic approach.
Bladder endometriosis, although rare, requires full-thickness excision when lesions penetrate the detrusor muscle. This necessitates precise dissection and multilayered closure (3,7). The involvement of the uterus by adenomyosis, further reinforces the need for total hysterectomy, which in this case was approached through a reverse technique after freeing lateral and posterior adhesions (8).
The rectosigmoid nodule required a segmental resection, facilitated by prior mapping. The use of fluorescence angiography with ICG confirmed perfusion of the anastomotic site and improved safety, as supported by recent studies promoting perfusion guided anastomosis in colorectal endometriosis (10).
One of the most critical aspects of this case was the identification and preservation of the inferior hypogastric plexus. As documented by Ceccaroni et al. and corroborated by Lanieri et al., neurogenic damage during parametrial or rectovaginal dissection can lead to postoperative bladder dysfunction, anorgasmia, and altered rectal continence (5,6). Therefore, nerve-sparing dissection of the Okabayashi and Latzko spaces was performed meticulously, aided by peripheral- to-central dissection to delineate avascular spaces before tackling fibrotic nodules (8,9).
The #ENZIAN classification in this case P0, O2/0, T3/1, A2, B1/1, C3, FA, FB, F1 reflected advanced multifocal disease requiring coordinated multidisciplinary surgery. This classification system proved especially useful in preoperative planning, surgical navigation, and postoperative documentation (9).
The integration of NOSE contributed to minimal invasiveness and improved postoperative recovery, eliminating the need for mini-laparotomy (11).
Conclusion
Triple-compartment laparoscopic excision for deep endometriosis is a safe and effective approach when performed by experienced surgical teams trained in nerve-sparing dissection, fluorescence-guided imaging, and reverse anatomical techniques. This case demonstrates that even in complex #ENZIAN stage C3 + FA/FB cases, complete excision with functional preservation is achievable.
The structured and standardized surgical strategy presented here promotes surgical reproducibility, reduced morbidity, and improved long-term quality of life for patients affected by this debilitating condition. Preoperative #ENZIAN mapping and intraoperative technological adjuncts such as ICG imaging and NOSE are critical elements of modern DE surgery.