Authors / metadata
DOI: 10.36205/trocar3.2021003
Abstract
Antigen cancer 125 (CA-125) is a hormonal marker for diagnosis of malignancy such as leiomyosarcoma and benign disease like endometriosis. In this case report, a 41-year-old woman with uterine bleeding, extremely high levels of CA 125 and large uterus presented to Nikan hospital with menorrhagia and anemia. Based on the clinical evidence of adenomyosis, magnetic resonance imaging (MRI) and transvaginal ultrasound (TVUS) were helpful in diagnosis. The uterus was enlarged and she had a preoperative CA-125 level of 4400 IU/ml. CA-125 level evaluation showed an increased level in a duration of 2 years before surgery. The enlarged uterus with increased CA-125 was suspect for leiomyosarcoma. Imaging investigations were helpful in making a definite diagnosis. A total laparoscopic hysterectomy, left salpingoophorectomy and right salpingectomy were done. Histopathology confirmed severe adenomyosis. The elevated CA-125 level returned to the normal range postoperatively. We preserved one ovary at this age, while if leiomyosarcoma were suspected before surgery, it was better to remove both ovaries. We report a case of adenomyosis with extra-ordinary raised CA-125 of 4400 IU/ml and sever menorrhagia.
Introduction
Antigen cancer 125 (CA-125) is a marker in the blood that is elevated in women with malignancies such as leiomyosarcoma, however it is elevated in people with other medical conditions and some healthy people. The normal blood concentration of CA-125 ranges from 0 to 35 U/ml which is based on the Bast et al. study in 1983 (1, 2). A definite cutoff value for endometriosis has not been determined because serum levels do not necessarily correlate with the severity of disease. Adenomyosis is a condition in which the inner lining of the uterus (the endometrium) invades into the muscle wall of the uterus (the myometrium). Adenomyosis is one of the diseases that can cause an elevated CA 125 level but the level which is reported in this case report is rarely reported. It is in various level in different studies.
As an illustration, the median CA125 levels for patients with adenomyosis was 102.1 (56.3-182.1) kU/L, in the Zhou et al study (3). Other investigations reported that in the differential diagnosis of adenomyosis and myoma, the cut-off serum level of CA125 with the highest accuracy (78.8%) and diagnostic value (61.2%) was 19 U/mL (4).
According to the fact that different imaging criteria are used, an accurate determination of its incidence or prevalence has not been carried out (1). The prevalence of adenomyosis ranges from 5 to 70 % (5-9). In the present study, trans-vaginalultrasound (TVUS) and magnetic resonance imaging (MRI) as well as the level of CA-125 make suspicious of adenomyosis before surgery. We describe a rare case of adenomyosis with Extra-ordinary raised CA-125 of 4400 IU/ml and sever menorrhagia.
Case report
A 41-year-old nulliparous woman with a BMI of 26.3 presented with several months of irregular, painful and heavy menses.
CA-125 level was as follow: 95.4 IU/ml, 2 years ago and 2500 IU/ml a year ago. The level of CA-125 in systematic blood test was 4400 IU/ml few days before surgery. Hemoglobin was 7.3 g/dl due to severe menorrhagia. The medical history was hyperlipidemia, hypertension and diabetes. She had a surgical history of appendectomy and myomectomy 12 and 6 years ago respectively.
Transvaginal sonography revealed the uterus has large size with dimensions of 171×104×32mm. Diffuse myometrial heterogenicity with cystic foci were seen suggesting diffuse adenomyosis. The endometrial thickness was 28 mm. In addition, there were 2 echogenic mass-like lesions measuring about 29×19mm and 15×20 mm in the upper part of the uterine cavity suggestive of endometrial polyps. An intramural to subserosal myoma measuring 95×68mm was seen in the lower segment of the anterior wall of the uterus. There was a subserosal myoma about 42×43mm observed in the right anterior wall. Both ovaries did have a normal size. Multiple echogenic foci were seen in the left ovary. There was no evidence of endometrioma or limited movement. Some free fluid was seen in posterior cul-de-sac, the left lower quadrant (LLQ) and the Morison pouch. Echogenic foci were seen in the uterine serosa and peritoneal surface of the posterior cul-de-sac possibly due to hemorrhage. There was no evidence of significant peritoneal seeding.
Abdominal and pelvic ultrasound demonstrated normal size of the liver and a diffusely increased echo-pattern in favor of fatty liver (grade 1). No dilatation was visualized in the common bile duct (CBD), and biliary tree, portal and hepatic vein. The Inferior vena cava (IVC) had a normal caliber. The gallbladder had normal wall thickness and echo-translucency. The pancreas and spleen had normal size and echogenicity. Both kidneys had normal shape, cortical thickness and cortico-medullary echogenicity. Both pelvicalyceal systems were not dilated. No mass lesion or detectable calculus was visualized.
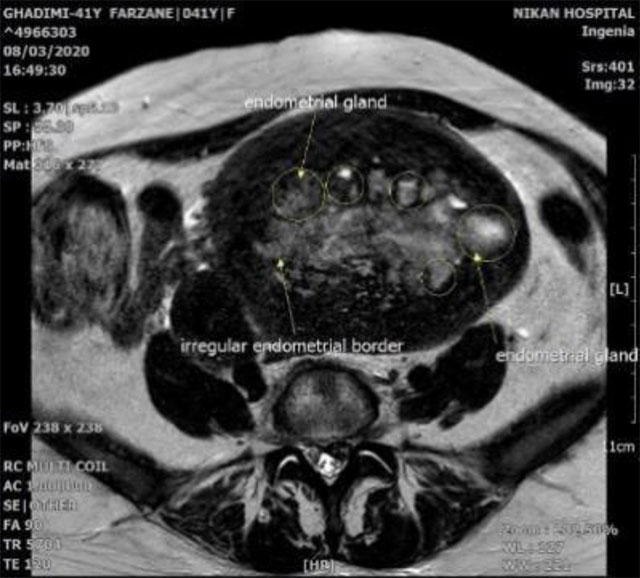
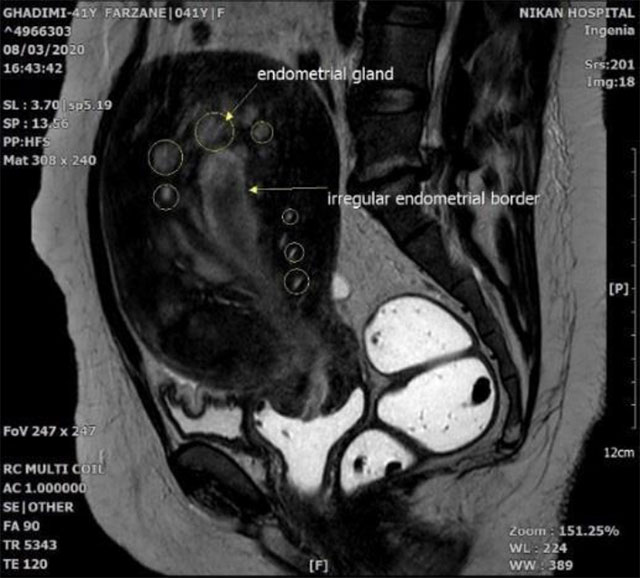
In addition, magnetic resonance imaging (MRI) showed an anterior synovial mass with a uterus in T1 weighted image (T1W), T2 weighted image (T2W) and diffusion- weighted imaging (DWI) sequences with mild enhancement after injection. Numerous oval and cystic lesions adjacent toward the endometrium were observed that caused the uterus to be enlarged. All masses were sub-endometrial. In addition, all sequences and the contrasts were similar to endometrium in various sequences (T1W, T2W, DWI, Apparent diffusion coefficient (ADC), T1+cont).
According to figure 1, position, signal and enhancement, diffuse adenomyosis and adenomyoma in uterine anterior wall were all suspicious. The focus of the hemorrhage around the ADC map of the endometrium was also marked by the presence of bleeding inside the lesion. The blooming artifact is marked.
Upper abdominal organs MRI with and without contrast were performed, Axial, coronal T1- T2/w images were obtained in addition: all evaluated organs were normal. Some free fluid was seen in the right sub-diaphragmatic region, Morison pouch and the pelvic cavity.
Serial serum CA 125 ranged from 95.4 from about 2 years ago to 4400 IU/ml before the surgery (Normal reference ranges = 0-35 IU/ml). (Chart 1). The imaging findings of TVUS accompanied with CA-125 level of 4400 IU/ml strongly suggested adenomyosis lesion.
Written consent form was obtained from patient. All procedures were performed under general anesthesia. The patient was administered two units of packed cells before surgery, which hemoglobin reaching to 9.5 g/dl. The patient was placed in the lithotomy position. Direct trocar insertion was performed above the umbilicus. The uterus was enlarged upto 20 week’s size and severe adenomyosis was observed.
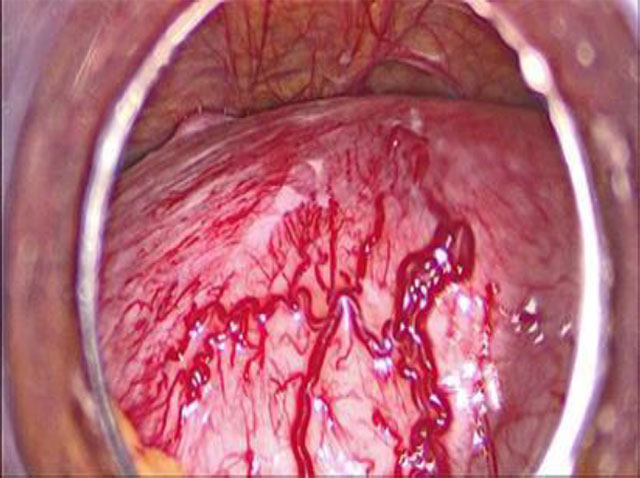
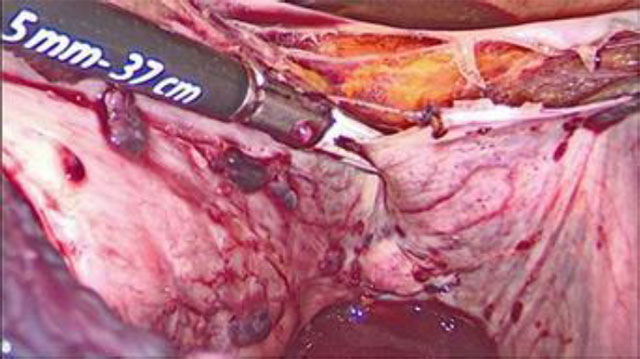
During laparoscopy, a hyperemic large uterus was observed in the pelvis. Endometriosis implants were observed in uterosacral ligaments and on the right side of the bladder and the peritoneum attached to the right ureter. Multiple vessels were observed on the surface of the uterine serosa and a manipulator was used to elevate the uterus from the pelvic floor. Uterus was large and heavy, therefore, moving the uterus with a manipulator was not easy, (Fig 2a,b).
Due to the possibility of heavy bleeding, ureteral dissection was performed and the origin of the uterine arteries and round ligaments were ligated on both sides. The bladder was dissected from the lower segment of the uterus and superior portion of the vagina. The vaginal wall was completely separated from the cervix above the uterosacral ligaments and vaginal fornixes delineated by the manipulator. The vaginal cuff was stitched with absorbable sutures. In bag morcellation was performed for uterus. Finally, total laparoscopic hysterectomy with left salpingoophorectomy and right salpingectomy was done. Adenomyosis was diagnosed by MRI findings and CA-125 level, therefore right ovary was preserved. Considering patient’s age and level of CA- 125, this case may be misdiagnosed with sarcoma and we had to remove the ovaries as it is suggested in various studies (10,11).
Macroscopic specimen at pathology revealed to be uterus, both fallopian tubes and left ovary and consisted of the morcellated material of the hysterectomy, a separated collapsed cyst and 2 fallopian tubes weighing 1284 gram in toto. The uterine cervix measured 3.5 ×2 cm the area enclosing external os measuring 1.4 cm in maximum dimension. The separated cystic structure measuring 5×2×2 cm, the internal surface was smooth and the wall thickness ranged between 0.2 and 1.5 cm, one fallopian tube measuring 5×0.9 cm, another one 6×0.8 cm a para tubal cyst was noted.
Right ureteral and uterosacral endometriosis, intestinal endometriosis, left uterosacral and urinary bladder endometriosis were observed. The endometrium consisted of several irregular soft tissue fragments mixed with large amounts of blood clots, a little muscle material measuring 5.5×2.5×5.1 cm in toto.
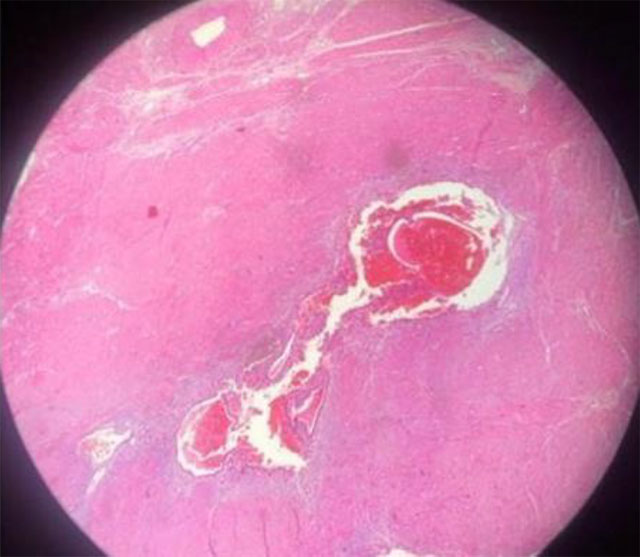
Microscopic findings revealed no evidence of malignancy and histopathology findings did support the final diagnosis (Fig 3): morcellated hysterectomy and bilateral salpingectomy and left ovarian cyst cystectomy (1284 gram). Chronic cervicitis with focal polypoid changes and Nabotyan cyst in the uterine cervix. A small intramural leiomyoma and adenomyosis was found in myometrium. An endometriotic cyst and cystic follicles were found in the left ovary. Para-tubal cysts were present in both fallopian tubes. There were superficial right ureteral endometriosis implants, right uterosacral and intestinal endometriosis was found, also left uterosacral and urinary bladder endometriosis. It is worth to mention here that the endometriosis implants in the pelvic were not deep implants.
There was no endometrial hyperplasia or malignancy detected in the operative specimen.
Endometrial curettage revealed large amount of blood clots containing few unremarkable endometrial and endocervical glands.
One month after operation CA-125 level were within normal range and the hemoglobin level gradually increased to reached to 10.6 g/dl. Histologically, the lesion proved to be extensive adenomyosis.
Discussion
The optimal cutoff values of serum CA125 for the pre-menopausal group and post-menopausal group was 162 U/mL and 75 U/mL, respectively. In this case, very high levels of CA-125 were observed in the absence of a malignancy.
The glycoprotein CA 125 can be secreted by several cells and enhanced by the inflammatory cytokines (12,13). Several cases of ruptured or unruptured endometrioma and adenomyosis have been reported with CA-125 concentrations as high as 9300 U/ml, (14) 6114 U/ml, (11)7900 U/ml, (12) 1796 U/ml (15) and 1138 U/ml (1). In a study Juang et al. in 2006 found that values of preoperative serum CA125 were significantly higher in the uterine leiomyosarcoma group than those in the uterine leiomyoma group. The optimal cutoff values of serum CA125 was 162 U/ml. These findings demonstrated that preoperative serum CA125 had a potential role in the differential diagnosis between early-stage and advanced-stage of uterine leiomyosarcoma. (16).
The finding of an incidental increased level of CA 125 challenges the diagnosis. Women with ovarian cancer often have an elevated level of CA 125, however it doesn’t always prove to be ovarian cancer. (17) A number of normal and noncancerous conditions can cause an elevated CA 125 level, including endometriosis, liver disease, menstruation, pelvic inflammatory disease, pregnancy, uterine fibroids. Adenomyosis is one of the diseases that can elevate the CA-125 serum level.
The cutoff of 35 IU/L for CA 125 was determined from the distribution of values in healthy individuals to include 99% of the normal population. However, the Mayo clinic reported that this normal value is less than 46 U/ml.
In a study by Pittaway et al. they found that mean CA-125 concentrations increased with severity from 17 ± 8 U /ml in mild disease to 51 ± 51 U/ml in severe endometriosis.CA-125 values more than 16 U/ml indicated endometriosis in 93% of the women. (18)
In the study by Skorstad et al. entitled ‘Sensitivity of different preoperative diagnostic tools used in women with uterine leiomyosarcomas’ he showed that CA 125 had low sensitivity for leiomyosarcoma, but in advanced stage disease high values were detected (19). Stage IV disease was present in 53.1% versus 25.5% (p = 0.01) of women with CA 125 values above 35 kU/L, compared with women with normal CA 125 values. Abnormal value of CA 125 was associated with more advanced disease.
Furthermore, in a study by Babacan et al they reported that abnormally high levels of CA 125 in 19.7% of patients with myoma, followed by significantly higher levels of CA 125 in patients with additional adenomyosis. The mean CA 125 were 27.3 ± 38.1 U/ml. Patients with additional pathologies, in particular with adenomyosis, had higher levels of CA 125 when compared with other patients (20). Furthermore, Takahashi et al. in their study reported that CA-125 level in patients with adenomyosis was 93.3 (21)
The accuracy of using only CA-125 testing for diagnosis is still limited. Serum CA-125 testing with accompanied imaging techniques can be done during initial screenings of women (4). This emphasizes the important role of MRI criteria to confirm the adenomyosis diagnosis in this case report.
MRI is a second-line examination in the diagnosis of internal adenomyosis that can differentiate the subtypes of adenomyosis. T2-weighted imaging showed the junctional zone (JZ) which separates the central endometrium (high signal intensity) from the outer myometrium (intermediate signal). Thickening of junctional zone exceeding 12 mm is the most common sign of adenomyosis.
Histologic diagnosis of adenomyosis include some criteria as follows: the presence of penetrating glands at least in one low-power field from the endo-myometrial junction or 2.5 mm below the basal layer of endometrium or deeper than 25% of overall myometrial thickness. Areas of myometrial smooth muscle proliferation are present around endometrial islands. This is the rare case report of adenomyosis of extremely elevated serum of CA 125 (of 4400IU/ml).
Conclusion
In conclusion, there is a diagnostic challenge of an extremely raised CA-125. Several disease may cause increased CA-125 level, while this case illustrates severe uterine adenomyosis which is associated with extremely elevated CA 125 levels. Imaging criteria for diagnosis of adenomyosis is helpful here.
Acknowledgments: We thank the whole staff of the imaging department of the Nikan hospital specially Dr. Saeed Nasiri.
Conclusion
The hysteroscopic view of cystic atrophy correlates 60 % with histopathology in the preliminary analysis. Cystic atrophy does not increase the risk of endometrial carcinoma, but presence of metabolic diseases may pose a risk of endometrial cancer due to the increased estradiol levels.
Hysteroscopic view: cystic atrophy. Immunohistochemistry may add value if the density of estrogen and progesterone receptors in the epithelial cells is high, in women with metabolic diseases and under evaluation for postmenopausal bleeding (9).
Limitations: The amount of endometrial tissue available for HPE varies in the two techniques of targeted biopsy and visual dilatation and curettage. Targeted biopsy with resection of the area or visual dilatation and curettage may increase the accuracy of the hysteroscopic view.
Strength: This is a prospective cohort ongoing study with a follow up of 12 months and aim to follow for 10 years.
Learning points: Evaluation for postmenopausal women must include a transvaginal ultrasound, hysteroscopy, as the technique involves direct vision, and an endometrial biopsy. Performing serum concentration of E2 may help in the management plan.
Sources / references
Fig 1a. hemorrhage around the ADC map of the endometrium is also marked by the presence of bleeding inside the lesion. Blooming artifact is marked.
Fig.1b. Adenomyosis in a 41-year-old woman. Sagittal T1-weighted image shows an enlarged uterus with homogeneous signal intensity. Sagittal T2-weighted image shows an ill-defined myometrial lesion of low signal intensity in the anterior myometrium.
Fig 2a. Hyper vascular uterus
Fig 2b. Endometriosis vesicular lesions in the posterior wall of the uterus
Fig 3 a Multiple locations of adenomyotic lesions in a uterus myomatosus.
Fig 3 b. 100×magnification photomicrograph of diffuse adenomyosis