Authors / metadata
DOI: 10.36205/trocar1.2023007
Abstract
By using the hysteroscope in contact mode to observe the endometrial vasculature, it became possible to correlate the appearance of the endometrial vasculature to the different phases of the human menstrual cycle This allows to date the endometrium and to compare the normal appearance of the endometrial vasculature with the endometrial vasculature in cases of pathology originating from the endometrium leading to physical complaints of the patients.
Introduction
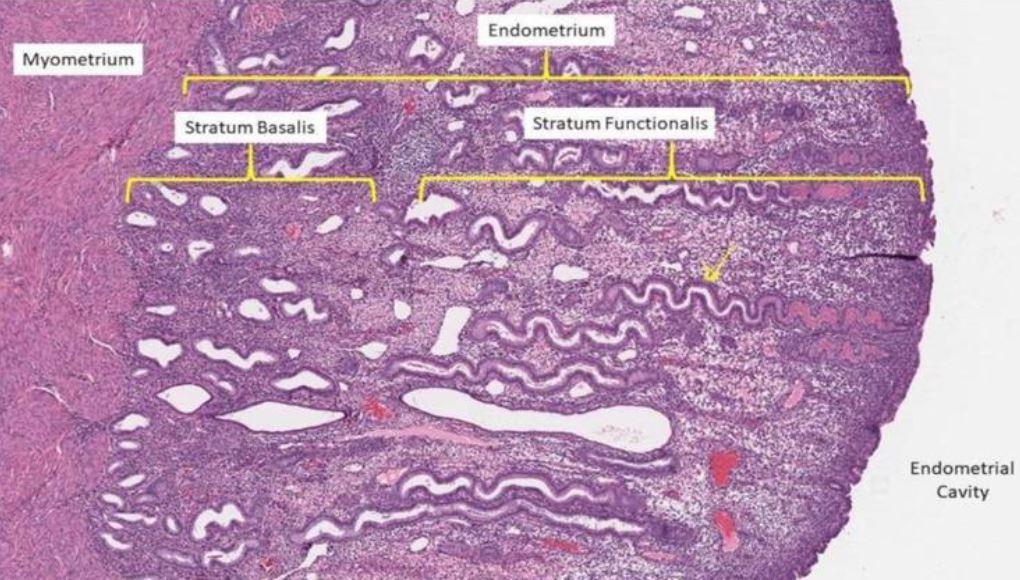
Endometrial blood vessels play an important role in the various physiological phases of the menstrual cycle. The presence of the specific vessels and their structure allows for identification of the different phases of the menstrual cycle corresponding with the histopathological dating (1-2). On the other hand, in different pathologies the appearance of the endometrial vessels is a primordial indicator like in Abnormal Uterine Bleeding (AUB), endometrial polyps, fibroids, polyps, adenomyosis, hyperplasia, endometrial related infertility and pill endometrium. Endometrial vessel angiogenesis allows to recognize the difference between vessel concentration and appearance in the basal and functional layers of the endometrium, congestion and dilatation as visualized by hysteroscopic examination (3). The recent scientific evidence that the vertical endometrial glands in the functional layer of the endometrium originate from a bed of horizontal glands in the basal layer of the same endometrium leads to the better understanding of the endometrial vessels both in the basal and the functional layer (fig 1). The vessels are feeding the glands by a network of side arborization from the main vertical vessel. The study proves that dating of the endometrium by observing the structure of the vessels is feasible and correlates with the histopathology of the biopsies.
Material Method
To identify the hysteroscopic appearance of vessels in the normal endometrium, the original study was performed at 200 consecutive hysteroscopies in consenting, healthy, patients in their fertile age, with normal menstrual cycles as evidenced by hormonal essays (estrogen, progesterone, FSH and LH) and recorded cycle days. In the final study 173 patients have been retained as 27 patients did stop during the study because complaints of too intense discomfort at hysteroscopy or irregular cycles. The patients presented with normal cycles and no complaints.
All hysteroscopies of the original study (1984-85)have been performed under CO2 dilatationof the uterine cavity at a pressure of 60 mm Hg (Richard Wolf GmbH Knittlingen Germany 2121 CO2 Metromat®). CO2 distention was used because at the time of the study this was the only existing method of distention available to perform hysteroscopy (1-2).
The hysteroscope used was a Hamou I Micro 30° for oblique, 6 mm diameter rounded hysteroscope (Karl Storz SE & Co KG Tuttlingen Germany) with magnification from 1:1 to 150:1 (1-2). For the study, an enlargement 1:60 has been used. Without prior dilatation, so as not to interfere with the endometrium, the hysteroscope was introduced in the uterine cavity and brought into contact with the endometrial surface on the anterior wall near the fundal area and the CO2 inflow was stopped. The image was focused and recorded (Olympus OM 2 camera, zoom 70-140 mm).
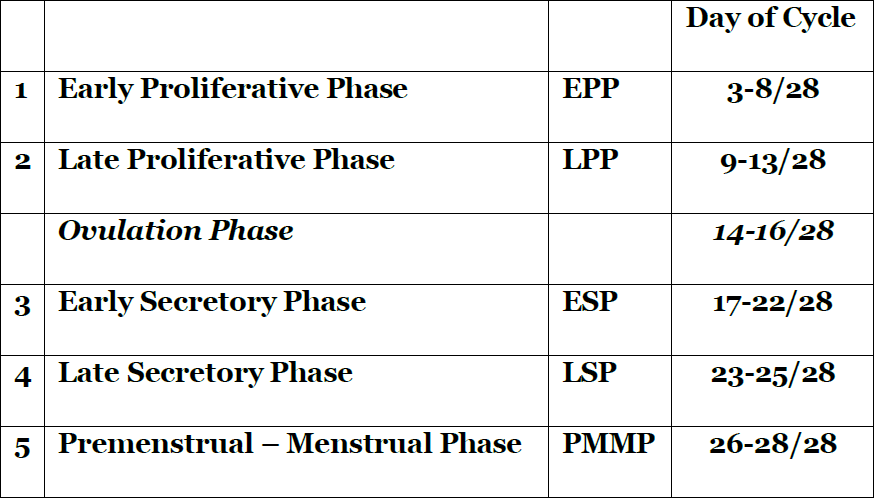
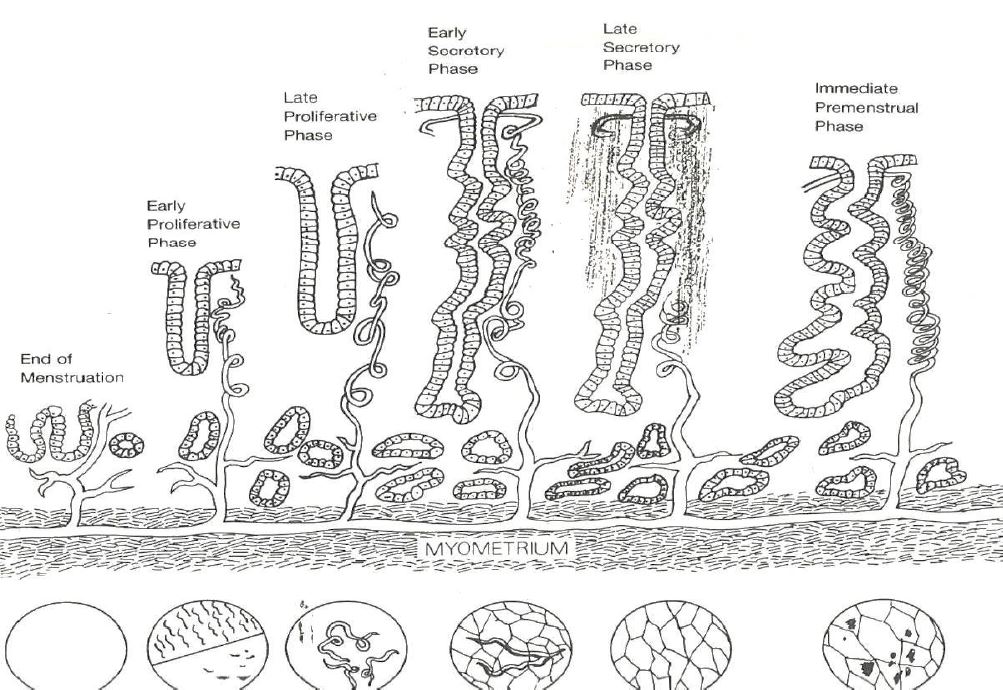
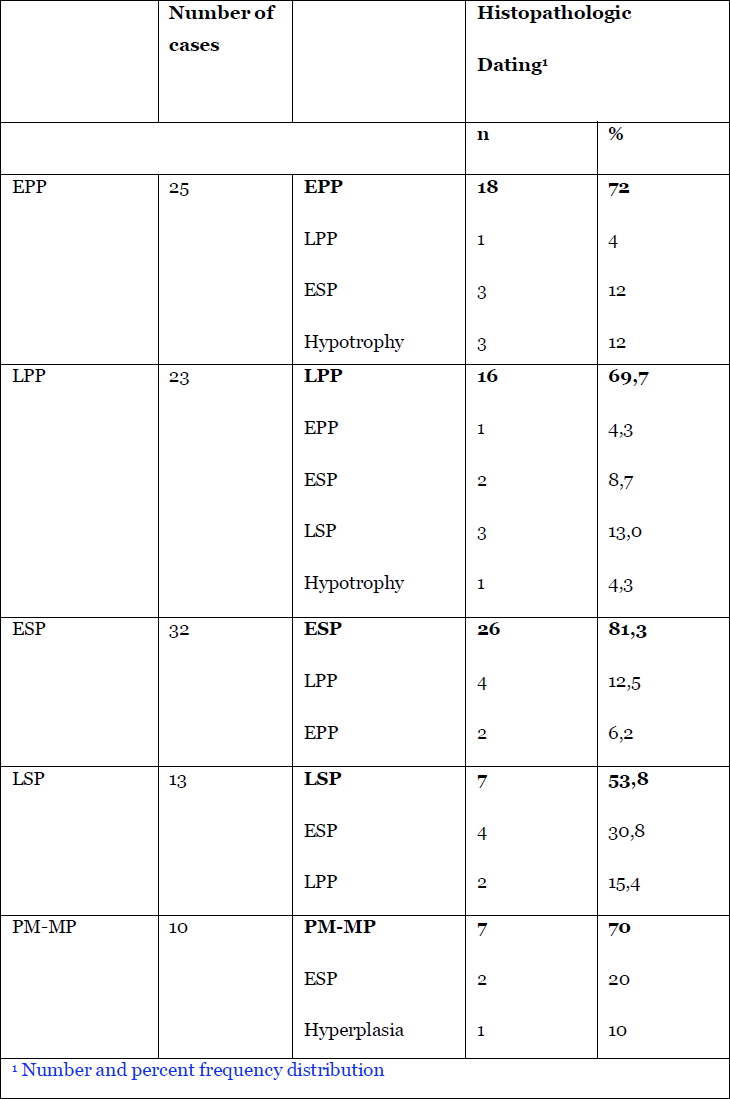
The performing hysteroscopist was blinded not knowing the exact phase of the cycle of the specific patient. Based on SRM Reynolds diagrammatic representation of the endometrial glands and arterioles, the arterioles throughout the menstrual cycle did allow a division of the endometrium into five periods by the visual impressions of the vessels (4)(Tab 1) (fig2). Initially the vascular patterncould be made out and the correspondence with the pathology of the original study is listed in Tab 2. Later fluid distention has been used and due to the fact that the endometrium with fluid distention is less compressed against the uterine walls dating of the endometrium by hysteroscope did become more accurate reaching an accuracy of around 96 % correspondence with pathology in expert hands. At the time of the study, historically, there was no need for Ethical Committees approval of the study.
Results
According to the visual impression through the hysteroscope correlated with the anamnestic, hormonal and pathology data, the human menstrual cycle could be divided in five stages:
1.Early proliferative phase (EPP) day 3-8/28.
This stage is divided in two sub stage the very early-stage day 1-3/28 and the later phase day 4-8.
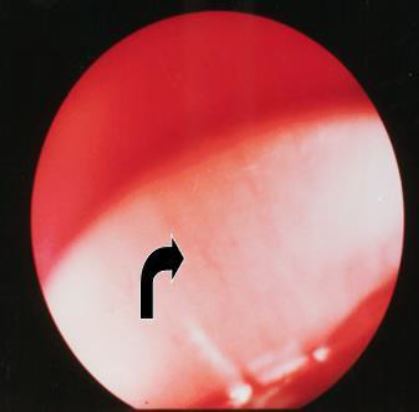
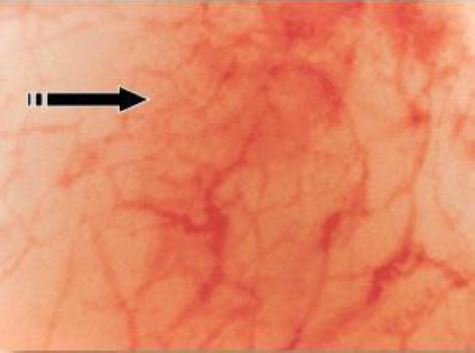
1.a) In the early stages blood can still be seen and there is a white reflection of the upper part of themyometrium though the basal endometrium – the endometrium that is not shedded during the menstruation – and the basal arterioles are visible running parallel with the myometrium and parallel to each other (Fig 3).
1.b) In the later EPP (day 4-8) the endometrium gains a pinkish colour due to the increase in heightnow it is the radial arterioles that grow vertically in the functional layer of the endometrium that are seen as comma like structures pointing upwards (Fig 4).
2.Late proliferative phase (LPP) day 9 – 13/28.
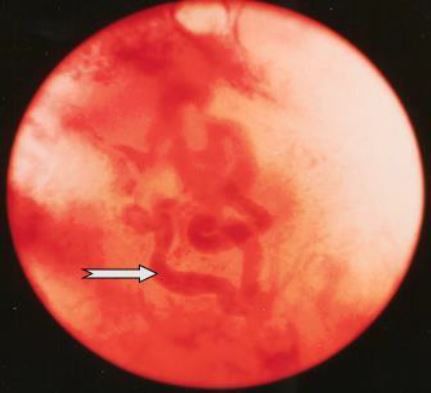
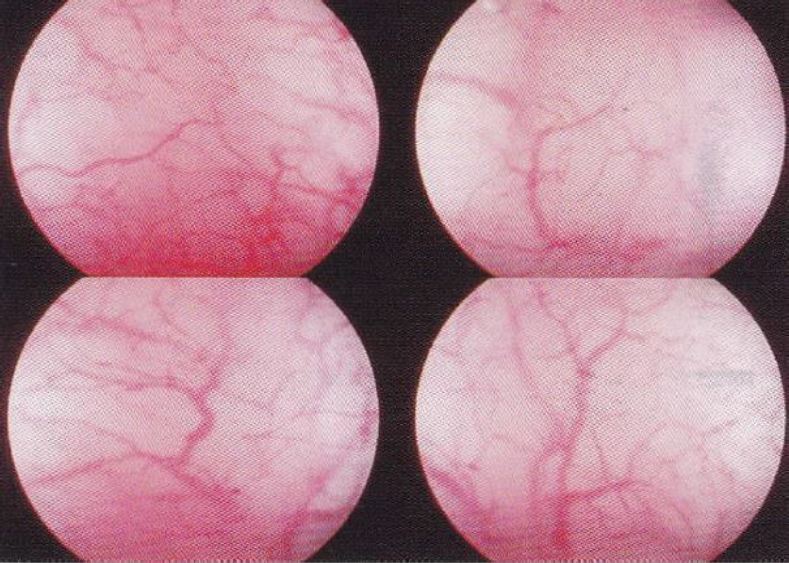
This is the stage where the coiled arteries grow off the radial arteries to grow alongside the endometrial glands to feed the latter. The anatomical aspect of these coiled arterioles – called spiral arteries in the original articles – can only be seen by pressing the hysteroscope into the growing functional layer of the endometrium. The overall colour aspect of the endometrium remains pale pinkish due to the extremely rapid growth in height of the functional layer of the endometrium (Fig 5).
3.Early Secretory phase (ESP) day 17-22/28.
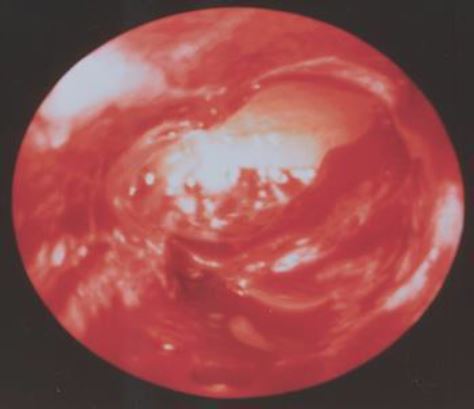
In this phase the endometrium is at its maximum height and due to the physiological oedema of the interglandular tissue it is transparent. Here the scope is able to visually interpret arterioles some 6 μ under the surface. The end part of the coiled arterioles can be seen whereas very small arterioles surrounding the glandular openings cross over these end parts. Moving the tip of the scope gently on the surface makes that these very small arterioles shift with the scope and the end parts of the coiled arteries remain in place giving rise to a two layered endometrium. The overall colour impression of the endometrium is very pale pink (Fig 6).
4.Late Secretory phase (LSP) day 23-25/28.
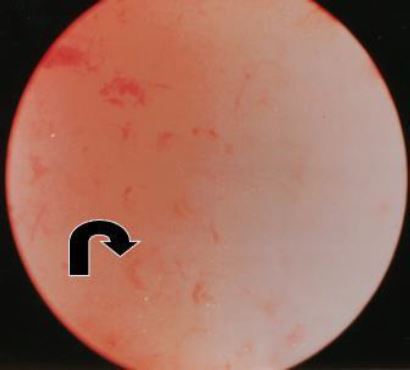
The densification of the intra glandular tissues of the endometrium, in this stage of the cycle, makes it impossible to visualize the coiled arterioles. The only vascular structures visible are the small arterioles around the endometrial glandular openings. The colour impression now turns to ivory (Fig 7).
5.Premenstrual – Menstrual phase (PM-MP) day 26-28/28
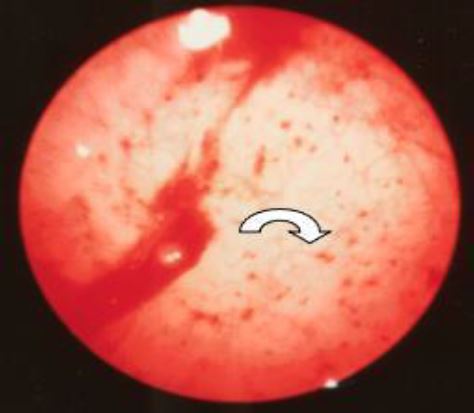
In this phase the contact hysteroscopy is no longer needed. On the surface of an ivory-colored endometrium red dots are observed (Fig 8). These do correspond by sub- epithelial bleedings. Due to the decrease in height of the endometrium the coiled arteries start to leak to form dissecting hematomas. These will then cause the shedding of the functional layer of the endometrium starting at the cornu in spiral like fashion (Fig 9).
Discussion
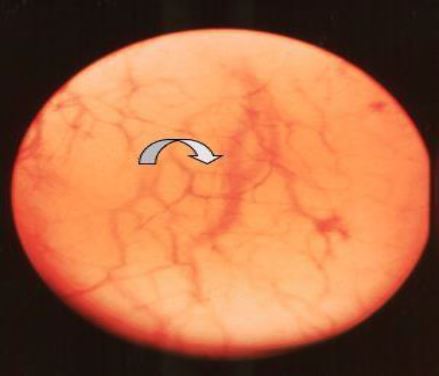
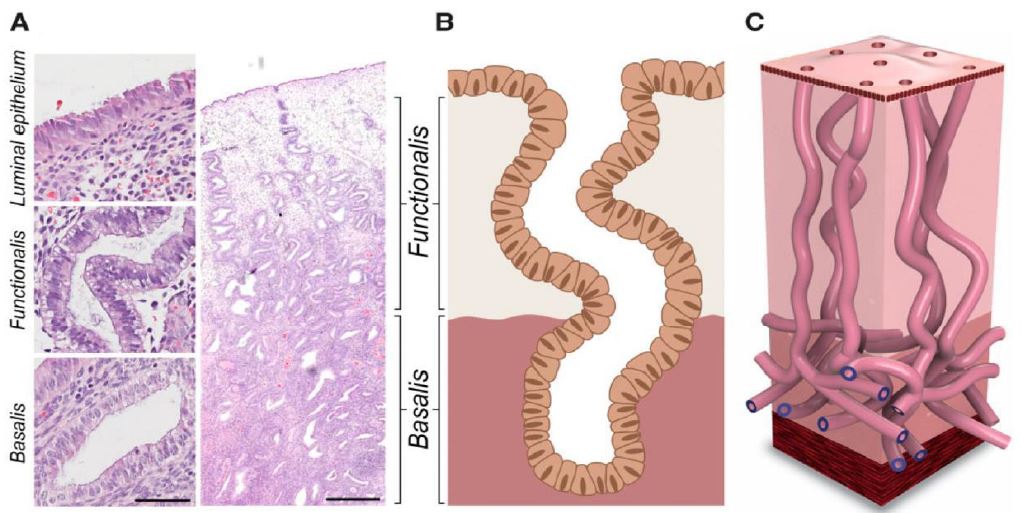
First of all, it has to be noted that pinpointing the ovulation phase by the visual impressions of the vessels trough the hysteroscope, the original scope of the study, averred to be an illusion due to the fact that hormonal changes do occur first and anatomical changes do follow the latter (5). This study did lead to the division of the cycle in five periods, phases, according to the visual appearance of the arterioles as described in the results, in correlation with the anamnestic data and the pathologic results obtained by direct biopsies through the hysteroscope (1-2). These results have been obtained by CO2 gas distention of the uterine cavity. This gas does induce hyperemia causing the arterioles to be better visible. In the later observations distention fluid has been used. This method of distention allows for the endometrium to “hang” into the uterine cavity allowing for a better detailed observation of the vessels and therefore for a more accurate interpretation (6). With the normal vasculature in mind, focus can be turned on interpreting the visual features of the arterioles of the endometrium in patients with endometrial abnormalities. Histopathology does describe changing in vessel concentration with a significant higher concentration in complex hyperplasia and pill endometrium whilst congestion and dilatation is significantly higher in patients with AUB (3) (Fig 10). These changes can be observed at hysteroscopy even without contact mode. The recent findings concerning the microanatomy of the endometrial glands does strengthen the results of the first studies as the “vertical” glands have a feeding vessel with lots of arborizations, the described coiled arterioles (7 – 8) (Fig 11).
There is also the need to harmonize the denomination of arterioles by both gynaecologists and pathologists in the different phases of the cycle.
Conclusion
Observing the endometrial arterioles during routine hysteroscopy does give valuable information concerning the phase of the cycle and even on possible symptoms as perceived by the patient.
However, as the studies on visualization of the arterioles in the endometrium have been performed in the period of CO2 distention, there is need for large clinical observational studies with liquid distention media.
References
Figure 1. Different layers: from myometrium over endometrium showing vessels and endometrial glands. Stratum Basalis remains during menstruation whilst the Stratum Functionalis is shed.
Table 1. Hysteroscopic dating of the endometrium according to the days of the cycle.
Figure 2. SRM Reynolds drawing of the endometrial growth to maximal height and decreasing height prior to menstruation with adaptation (BvH 1987) on densification of the interglandular tissues and schematic corresponding representation of the hysteroscopic view in lower part.
Table 2. The numbers of the dating by hysteroscopy and by histopathology. The dating as given by the blinded hysteroscopists and the respective corresponding histopathology diagnoses and percentages are listed.
Figure 3. Early Proliferative Phase (CD 01-02). There is still blood in the cavity. The light is reflected on the basal layer of the endometrium. Basal arteries are seen running parallel with each other in the horizontal plane. (CO2 distention 60 mm Hg).
Figure 4. In the later phase of the Early Proliferative Phase (CD 3-8) the arcuate arteries are growing to the lumen of the uterine cavity – vertical as opposed to the basal arteries. These arterioles are seen as pointing upwards. (CO2 distention 60 mm HG)
Figure 5. Late Proliferative Phase (CD 09-13). The main features are the coiled arterioles developing as the continuation of the radial arterioles. Only seen when penetrating the functional endometrium with the hysteroscope. (CO2 distention 60 mm HG)
Figure 6. Early Secretory Phase (CD 17-22). The two layered endometrium. The final part of the coiled arteriole is seen and the fine maze of the arterioles surrounding the endometrial gland openings are seen running over the coiled arterioles. (CO2 distention 60 mm HG)
Figure 7. Late Secretory Phase (CD 23-25) Due to the densification of the interglandular tissues the final part of the coiled arteries is no longer visible. The arterioles surrounding the glandular openings are prominent in view. The colour becomes more ivory. (CO2 distention 60 mm HG)
Figure 8. Premenstrual and Menstrual Phase (CD 26-28). Due to the shrinkage of the interglandular tissues the coiled arteries begin to leak and form dissecting hematomas inducing the shedding of the functional layer of the endometrium. The blood collections seen are not in the glandular openings but in the interglandular tissues. (CO2 distention 60 mm HG)
Figure 9. Menstruation. The fundus is already denudated. Remark the patches of functional endometrium still present whilst the endometrium is expulsed in a spiral fashion starting at the cornual parts of the uterine cavity. (CO2 distention 100 mm HG)
Figure 10. Pill endometrium. The very pink colour is due to an increased numeric presence of vessels, in a significantly higher concentration than in the normal.
Figure 11. Architecture of human endometrial glands: past and present: (A) Hematoxylin and eosin-stained human endometrial section at x400 and x 20 magnification (scale bars= 50 and 500 μm, respectively). (B) 2 D scheme of the pre–2020 consensus view of endometrial glandular architecture, with functional glands running a vertical course to the basalis gland and terminating in blind pouches. (C) 3D scheme of the novel endometrial gland arrangement based on recent findings, with basalis glands exhibiting a branching, mycelium like configuration running perpendicular to functionalis glands (Tempest N, Hill C J, Maclaen A, Marston K, Powell SG, Al-Lamee H, Hapangama D.Novel microarchitecture of human endometrial glands: implications in endometrial regeneration andpathologies. Hum Reprod Update 2021;28(2):153-171)