Authors / metadata
DOI: 10.36205/ trocar5.2024005
Abstract
The author recalls how microcolpohysteroscopy is a technique more than forty years old that allows to observe the cells of the uterine cervix “in vivo”, without the need to carry out a bi-opsy and send it to the laboratory for a diagnosis. For many years he has been using pre-operative microcolpohysteroscopy to precisely establish the margins of the lesion to be re-moved, and obtain a minimally invasive surgery. Two cases are presented in which a peri-operative microcolpohysteroscopy is performed on the surgical cone, to evaluate – in the op-erating room – whether the excision was adequate and did not leave residual lesions on the uterine cervix.
Histology on the removed cervical cones confirms (after a few days) the peri-operative diagnosis, thus demonstrating the effectiveness of using this method for a tailor-made surgical excision.
Introduction
The Micro-colpo-hysteroscope by Storz GmBH was created in 1980 based on a design by Dr. Jacques Hamou 1 and put on sale in July 1981. The instrument, over the years, has undergone some modifications to make it more manageable, and has the characteristics of a hysteroscope, a colposcope and a microscope capable of magnification up to a maximum (currently) of 80x. (Fig. 1) Several studies have highlighted how it is possible to observe preneoplastic lesions induced by the Human Papilloma Virus (HPV) on the uterine cervix, and their possible extension within the cervical canal 2, 3, 4 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 .
Unfortunately, the method practiced by a few specialists around the world did not have the diffusion it deserved. The main reason lay in the fact that the micro-colpo-hysteroscopic diagnosis requires the skills of the cytologist (able to recognize and diagnose the cells of the uterine cervix) and the gynecologist (who -unlike the cytologist in the laboratory – seesand visits the patient in his own office).
Following the limited diffusion and consequently the reduced amount of instruments sold, the manufacturing company ceased production of the Hamou micro-colpo-hysteroscope type I, and subsequently of the type II, replacing both with a reduced caliber instrument (2.5 mm ) which makes seeing the cells slightly more difficult. This inconvenience can be overcome by using an endoscopic camera equipped with a zoom, which allows a visual increase on the screen such as to allow a vision comparable to that of the old models.
Currently, the distribution of the Hamou type III micro-colpo-hysteroscope is not widespread throughout the world, and this explains the difficulty in purchasing it for those gynecologists interested in using it in their clinical practice. For those who own a Micro-colpo-hysteroscope, or have the opportunity to purchase one, I remind of the standard procedure for obtaining an excellent view of the cells directly “in vivo” on the patient.
In addition to the Hamou micro-colpo-hysteroscope, it is necessary to have 5% Lugol’s solution and Waterman’s blue ink, the only elective vital dye for the squamous epithelium of the uterine cervix. After having exposed the uterine cervix with a common vaginal speculum, as if carrying out a cytological sample, the epithelial surface must be cleaned of mucus with a cotton ball soaked in physiological solution (never use acetic acid as it would cause edema of the mucosa and would prevent subsequent observation of the cells!)
We then proceed with a close observation without any staining, and – after the application of Lugol’s solution – the same procedure will be repeated to observe any iodo-weak areas. Obviously, these will be the areas where the cellular elements will be evaluated most carefully.
The application of Waterman’s Blue ink (able to color only the elements of the squamous epithelium or those of squamous metaplasia, leaving the cylindrical cells colorless), allows the subsequent observation of the cells in contact. This means that by placing the distal lens in contact with the epithelium, and acting on the knob for correct focusing, we have the possibility of seeing the same images as the cytologist when observing a slide, but with the enormous advantage of knowing the location and extent, in case of cytopathic alterations induced by the human papillomavirus.
Material and methods
We report two cases recently sent to our attention, in which a micro-colpo-hysteroscopic diagnosis of High-Grade Intraepithelial Lesion (HSIL) was made, and subsequently subjected to surgical removal with loop.
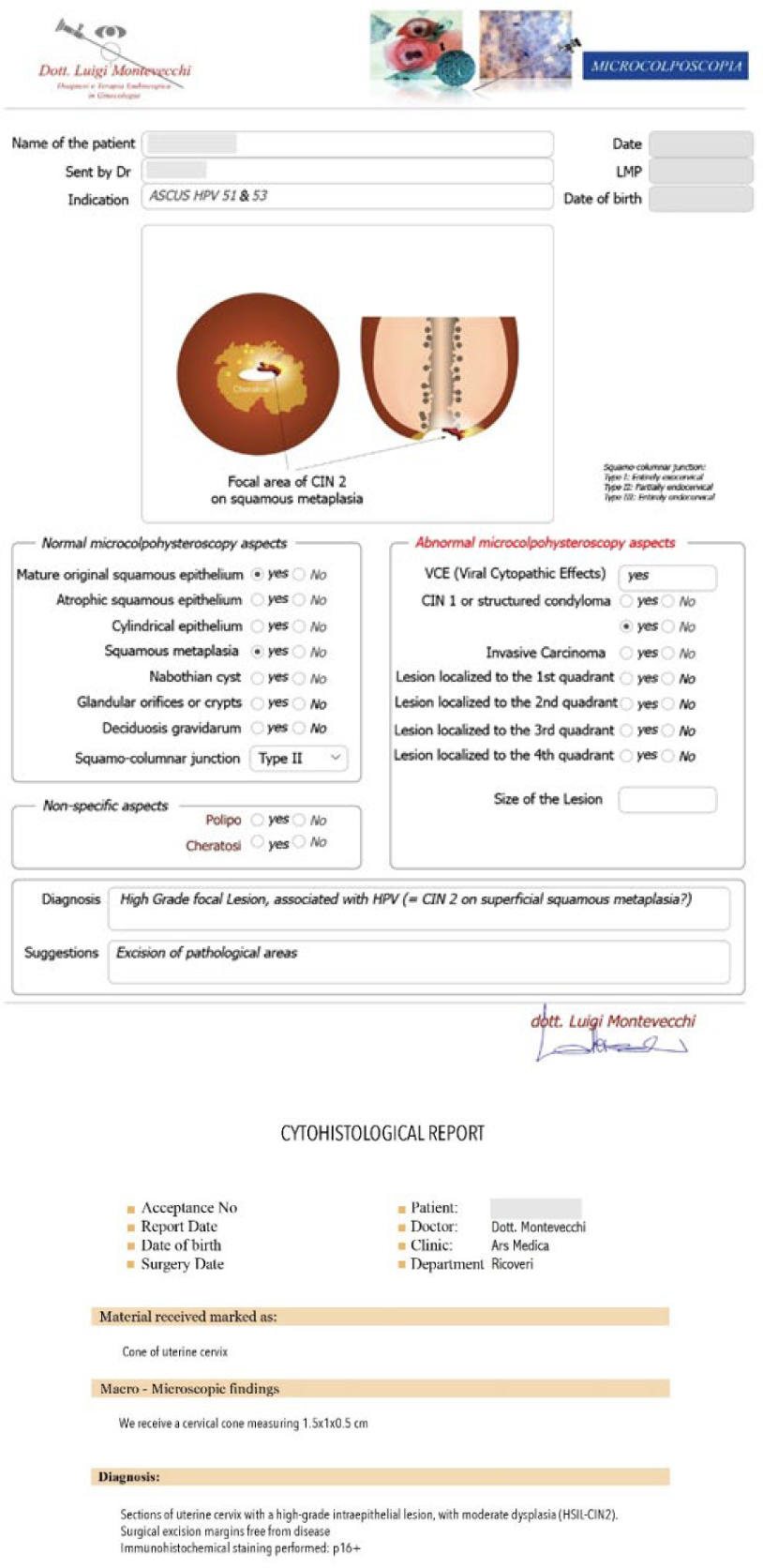
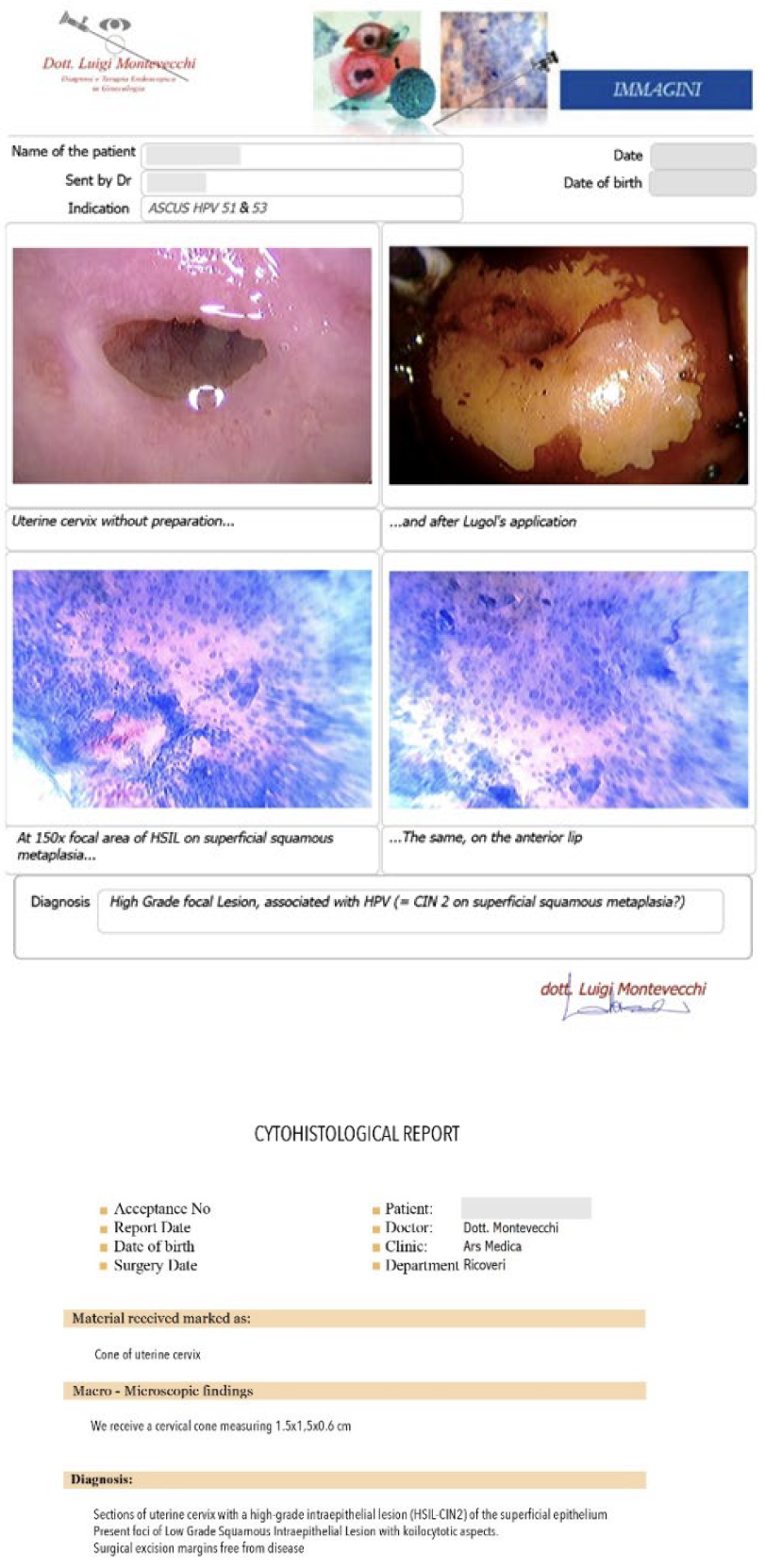
Case #1: 26-year-old patient, with cytological diagnosis of ASCUS, and positivity for HPV 51 and 53. The Microcolpohysteroscopy highlights the presence of a High Grade Inraepthelial Lesion (HSIL), not detected by the cytological examination, and therefore we suggest excision with loop of the pathological areas (Fig. 2)
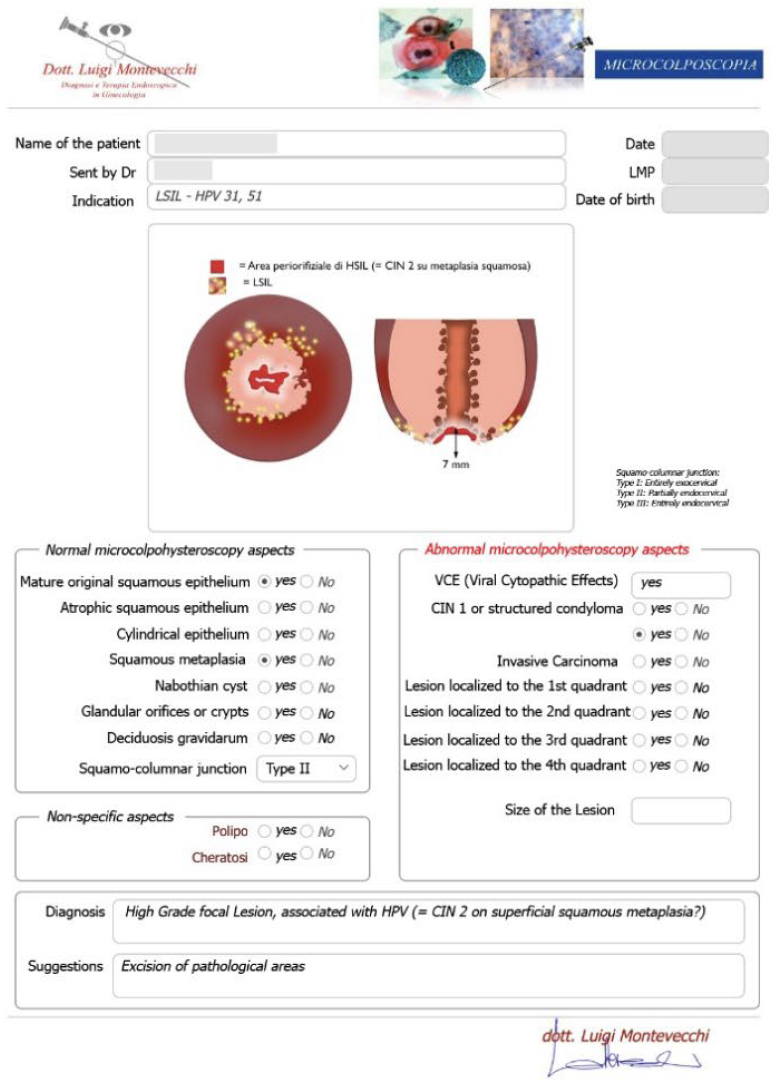
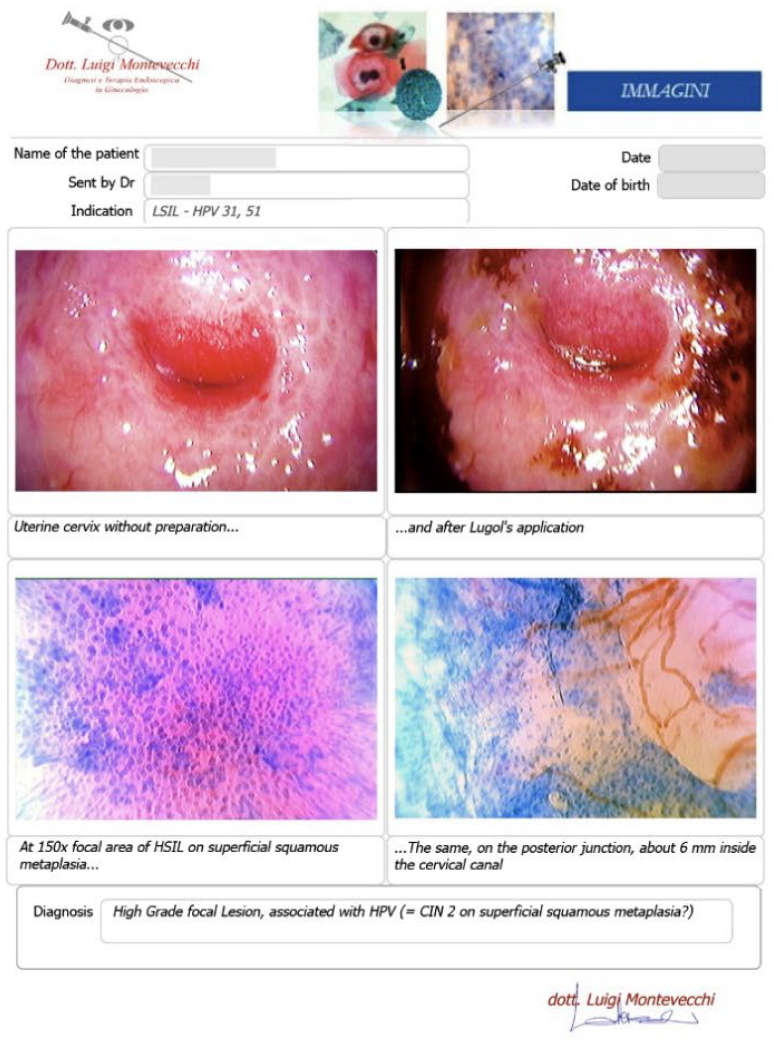
Case #2: 30-year-old patient, suffering from multiple sclerosis diagnosed at the age of 17, currently being treated with Mavenclad ® (cladribine: chemotherapy and immunosuppressant drug used for the treatment of hairy cell leukemia and relapsing-remitting multiple sclerosis) after months of treatment with Cortisone. Indication for Microcolpohysteroscopy was cytological diagnosis of LSIL and positivity for HPV 31 and 51. The exam highlights a High-Grade lesion (HSIL) and excision of the pathological areas is suggested (Fig. 3) Few weeks later both patients are placed in the operating room, under general anesthesia with Propofol ®; genitals are disinfected and the location and extent of the alterations attributable to HSIL are checked once again. After the examination, we proceed with local infiltration with epinephrine diluted 1:200,000 for preventive hemostatic purposes, and a loop of the appropriate size is chosen (not too small nor too large), to carry out a tailor-made excision. Immediately after the excision, micro-colpo-hysteroscopy is repeated on the margins of the uterine cervix, and on the removed cervical cone, to verify that they are free from the disease (Fig. 4) Once the presence of external and internal margins free from the disease has been ascertained, it is possible to proceed with the coagulation of the bleeding vascular nozzles, without having to repeat a deeper or wider excision (Fig. 5).
Results
Both patients underwent a “tailor-made” excision of the pathological areas, so as to avoid the risk of excessively wide or deep, or insufficient removals, with the risk of leaving residual HSIL areas on the cervix. The histological examination provided by the laboratory fully confirmed both the diagnosis (HSIL = CIN 2) and the accuracy of the excision, reporting the disease-free margins (Fig. 6)
Discussion
Microcolpohysteroscopy has for years been used almost exclusively as a diagnostic investigation to confirm or exclude the presence of a preneoplastic lesion on the uterine cervix, and to identify its margins so as to send this precious information to the surgeon who will have to perform the excisional surgery. This article proposes to use the technique in the operating room, immediately before and immediately after the use of the electric loop to remove the pathological tissue, and to verify its effectiveness.
Excisional treatment of high-grade cervical lesions is normally carried out using an electric loop whose dimensions depend on the exocervical extension of the pathological areas observed on the exo-cervix using conventional colposcopy.
Unfortunately, this procedure can have two drawbacks: the macroscopic observation of the external limits of the observed lesion cannot have the same accuracy as the microscopic vision, capable of establishing the external limits of the cellular alterations to be removed, and secondly the colposcopic observation does not can accurately determine the endocervical extent of the lesion. As a consequence, it is not uncommon to obtain an incomplete excision of the pathological tissue, with the risk of having to repeat the treatment again due to the persistence of the disease.17,18
On the contrary, especially in initial high-grade lesions, standardized excisional treatment can remove an excessive amount of tissue, and this is not desirable in young subjects with the desire to become pregnant.
Personalization of treatment is the solution to the problem. Immediately before choosing the size and shape of the loop, an accurate micro-colpo-hysteroscopy is carried out, with the patient on the operating bed. Once the external and endocervical margins have been established, the loop is chosen. The excision takes place with a decisive and progressive passage of the loop in an antero-posterior or lateral-lateral direction, depending on one’s habits, and immediately after the removal of the fragment of cervix, a new microcolpohysteroscopic check is carried out on the margins of the residual cervix, and on the removed piece, applying Waterman’s blue solution again, to be able to observe the cells. If pathological cellular elements are observed on the margins of the uterine cervix or of the removed cervical cone, it will be possible to proceed with a slight enlargement of the excision, thus avoiding learning of the incomplete excision only following the histology report, having to repeat a new hospitalization for the patient to complete the surgical treatment.
While the advantages of this method appear evident, the only disadvantage is linked to the difficulty of acquiring the instrument or learning the technique, which unfortunately is still reserved for a few specialists today.
Conclusion
The use of preoperative micro-colpo-hysteroscopy to precisely identify the tissue to be removed allows for a “therapeutic” conization to be obtained, enormously reducing the risk of carrying out an incomplete or excessive excision Furthermore, the immediately postoperative micro-colpo-hysteroscopic control on the margins of the uterine cervix allows an immediate evaluation of the total removal thus reducing the risk of persistence of the lesion.