Authors / metadata
DOI: 10.36205/trocar5.2025008
Abstract
Introduction: Adenomyosis is a chronic gynecological disorder characterized by invasion of endometrial glands and stroma within the myometrium, frequently resulting in debilitating pelvic pain, menorrhagia, and impaired fertility.
Objective: This study aims to evaluate the safety, efficacy, and clinical outcomes of ultrasound-guided microwave ablation (MWA) to manage diffuse adenomyosis in a cohort of 100 patients, focusing on symptom relief, uterine volume reduction, and complication rates.
Method: A prospective, single-arm clinical study of 100 women aged 28–50 years with diffuse adenomyosis was conducted. All patients underwent MWA under general anesthesia with ultrasound guidance. Symptom severity was measured using the Visual Analog Scale (VAS) for pain and the Pictorial Blood Loss Assessment Chart (PBAC) for menstrual bleeding. Uterine volume was evaluated using transvaginal ultrasound. Follow-up assessments were conducted at one, two-, and six-months post-procedure.
Results: At 6 months, dysmenorrhea VAS scores showed an average reduction of 78%, and 92% of patients reported significant improvement in menorrhagia. The average reduction in uterine volume was 30% at 2 months and 40% at 6 months. No major complications were observed. Minor adverse effects included transient pelvic discomfort in 6% of cases.
Conclusion: Ultrasound-guided MWA is a minimally invasive, uterus-preserving intervention that demonstrates significant symptom relief and reduction in uterine volume in patients with diffuse adenomyosis.
Introduction
Adenomyosis is a benign uterine disorder characterized by the infiltration of endometrial tissue into the myometrium, leading to a diffusely enlarged uterus and the disruption of normal uterine architecture. Clinically, it presents with symptoms such as dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and, in some cases, subfertility or infertility. The condition is estimated to affect up to 20–30% of women in the general population and is most commonly diagnosed in women between the ages of 30 and 50 (1). However, recent advancements in imaging have shown that adenomyosis may be underdiagnosed, especially in younger women and those with overlapping symptoms of fibroids or endometriosis. Traditionally, the gold standard for diagnosing adenomyosis was histopathological evaluation following hysterectomy. However, the advent of high-resolution transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI) has enabled non-invasive diagnosis with increasing accuracy. On ultrasound, features such as myometrial cysts, a heterogeneous myometrium, and a thickened junctional zone are commonly used criteria (2). Management options for adenomyosis are typically categorized into medical, surgical, and minimally invasive therapies. Medical treatment primarily includes hormonal agents such as progestins, combined oral contraceptives, and GnRH analogs (3). While these treatments may provide temporary relief, symptoms frequently recur upon cessation. Definitive surgical treatment by hysterectomy remains a commonly performed procedure, particularly in women who have completed childbearing. However, hysterectomy is not an ideal solution for women who wish to preserve fertility or avoid major surgery (3,4). Minimally invasive techniques such as uterine artery embolization (UAE) and high-intensity focused ultrasound (HIFU) have emerged as uterus-preserving alternatives. Although effective in certain cases, UAE may result in uterine ischemia, while HIFU is limited by the depth and vascularity of the lesion, operator experience, and patient selection (5). Microwave ablation (MWA) has gained attention as a newer thermal ablation modality. MWA utilizes electromagnetic energy to create localized tissue necrosis through dielectric heating (5). Unlike radiofrequency ablation, MWA allows higher temperatures over larger volumes and is less affected by tissue impedance. This technique has been well studied in liver and renal tumors and is now being adapted for gynecological applications. This study evaluates the application of ultrasound guided MWA in a cohort of 100 women diagnosed with diffuse adenomyosis. The aim is to provide real-world data on clinical outcomes, including symptom relief, uterine volume reduction, and procedural safety, thereby supporting its role as a viable alternative to hysterectomy.
Material and Methods
1.1 Study Design and Participants
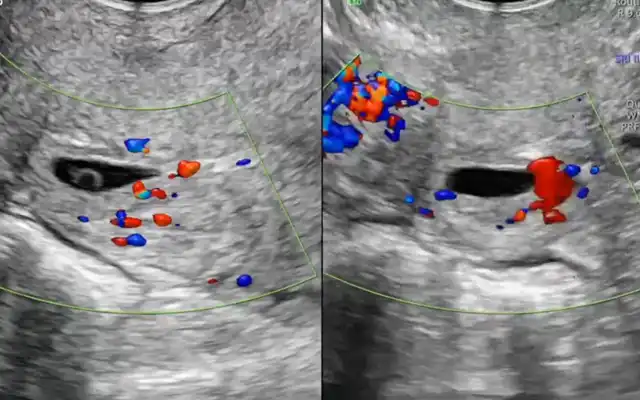
This prospective observational study was conducted at a tertiary care center between January 2022 and June 2023. A total of 100 women aged between 28 and 50 years with clinically and radiologically confirmed diffuse adenomyosis were included. Diagnosis was based on transvaginal ultrasound (TVUS) findings such as a globular uterus, asymmetrical myometrial thickening, myometrial cysts, and hyperechoic striations. Patients provided informed consent and ethical approval was obtained from the institutional review board.
1.2. Inclusion and Exclusion Criteria
Inclusion criteria were:
- age between 25 and 50 years
- symptomatic diffuse adenomyosis refractory to medical treatment
- no desire for immediate conception
- willingness to undergo general anesthesia
Exclusion criteria included:
- focal adenomyosis or concurrent large fibroids (>5 cm)
- suspected malignancy
- pregnancy
- uterine length on ultrasound more than 12 cm
- contraindications to general anesthesia.
1.3. Microwave Ablation Protocol
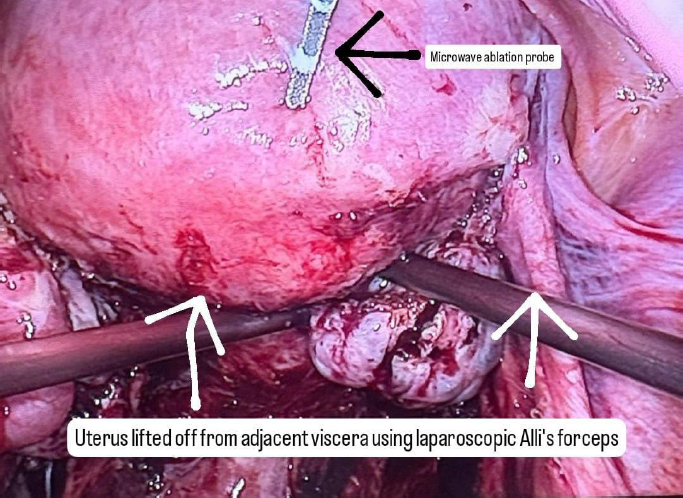
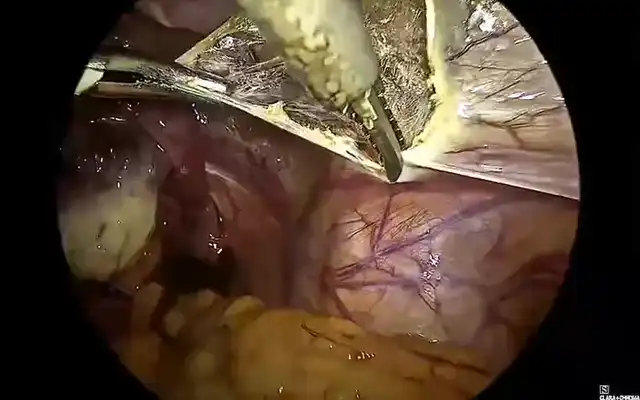
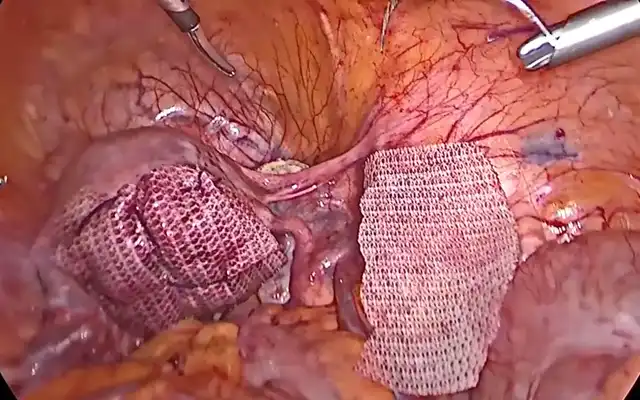
A careful selection of symptomatic patients with diffuse uterine adenomyosis was made. Under general anesthesia, patient was placed in lithotomy position and standard laparoscopic port entry was mad e. Intraoperative picture was assessed. Areas of most uterine density were assessed laparoscopically. Simultaneously, an intraoperative transvaginal ultrasound probe was inserted to map the areas of adenomyosis. Under USG guidance, the microwave ablation needle was inserted into the uterus percutaneously and the hyperechoic needle tract followed on USG to ensure that the needle is well away from the endometrium. Once the desired area is reached, ablation power was set between 40–60 W (rarely, up to 80 watts in case of diffuse adenomyosis), and each cycle lasted 5-7 minutes depending on the lesion size and impedance feedback. Multiple overlapping ablations were performed as needed to cover the entire affected area. A period of probe cooling was maintained in between ablating two regions wherein probe temperature was brought back to 18 degrees Celsius using an internal circulation (within the ablation probe) of chilled normal saline. Throughout the ablation procedure, temperature was continuously recorded on the Canyon device and ablation was stopped if temperatures reached above 30 degrees. Ice packs and saline irrigation were used to protect adjacent organs from thermal spread. Throughout the procedure, the uterus was lifted up from the adjacent bowel and viscera, supported by two Alli’s forceps inserted through the two lateral ports.
The MWA system utilized was Canyon Medical Inc. KY-2000A, operating at a frequency of 2.45 GHz with power output adjustable up to 100 W.
1.4.Postoperative Management and Follow-Up
Patients were observed for 24 hours post-procedure. Approximately, 10% of patients complained of cramping post-operative pain. Non-steroidal anti-inflammatory drugs (NSAIDs) were administered for pain control. Follow-up was scheduled at 1, 3, and 6 months. Assessments included:
- Pain assessment using the Visual Analog Scale (VAS)
- Menstrual blood loss quantified via Pictorial Blood Loss Assessment Chart (PBAC)
- Uterine volume calculated using ultrasound: V = 0.523 × L × W × H
- Complication monitoring (e.g., infection, thermal injury)
Symptom improvement was defined as a ≥50% reduction in VAS or PBAC scores. Imaging was performed at each follow-up visit to assess volume changes and resolution of adenomyotic zones.
Results
2.1. Patient Demographics and Baseline Characteristics
The study included 100 women with a mean age of 38.4 ± 5.7 years. The average duration of symptoms prior to intervention was 3.6 ± 1.2 years. All patients reported dysmenorrhea, and 92% reported menorrhagia. The average baseline VAS score for pelvic pain was 8.2 ± 1.1, while the mean PBAC score was 365 ± 54. Mean uterine volume at baseline was 180 ± 45 cm³.
2.2. Procedural Details
MWA was successfully performed in all 100 patients (technical success rate: 100%). The mean number of ablation zones was 4.2 ± 1.1, and average ablation time was 26 ± 7 minutes per patient. No intraoperative complications such as bowel injury or uterine perforation were encountered.
2.3. Clinical Outcomes
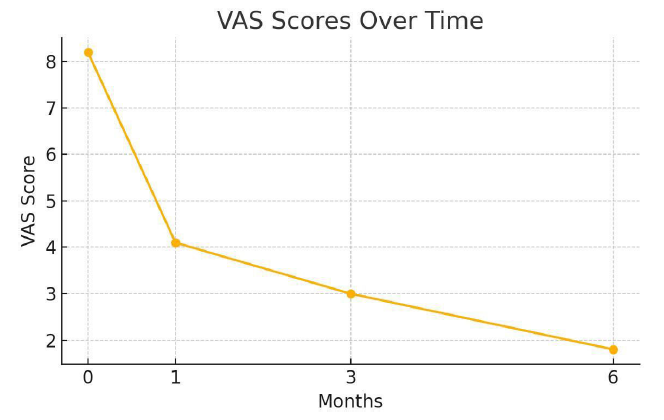
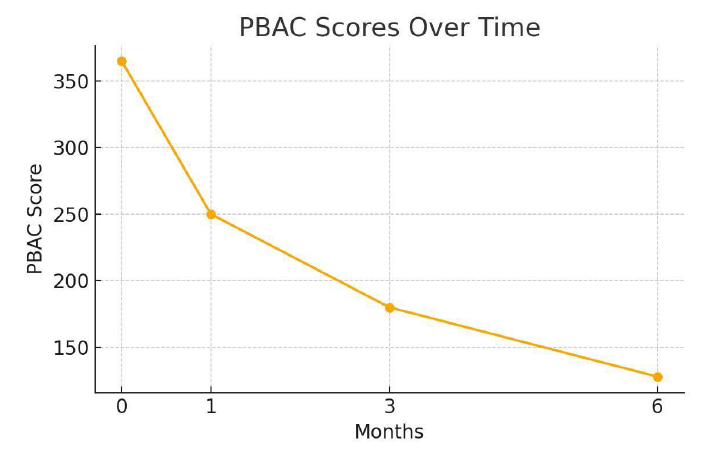
At 1-month follow-up, VAS scores decreased to 4.1 ± 0.9 and continued to decline to 3.0 ± 0.7 at 3 months and 1.8 ± 0.6 at 6 months. PBAC scores showed parallel improvements, with a 65% reduction observed by 6 months. At 6 months, 92% of patients had at least a 50% reduction in symptom severity, with 67% reporting near-complete resolution of dysmenorrhea.
2.4. Uterine Volume Reduction
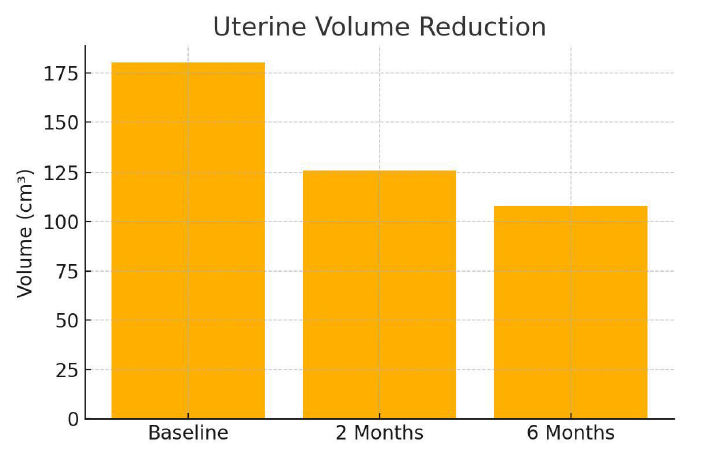
Mean uterine volume decreased by 30% at 2 months and 40% at 6 months. No patient required a repeat ablation or surgical intervention during the follow-up period.
2.5. Complications and Safety
No major complications were reported. Around 10% of patients complained of cramping post-operative pain during immediate post-op Day 1 which was easily resolved with mild dose of analgesics like NSAIDs. Minor adverse effects were noted in 6% of patients, including transient pelvic pain and low-grade fever. These resolved spontaneously or with symptomatic treatment within 48–72 hours. Around 17% of patients reported mild watery discharge vaginally up to one month post-surgery which could potentially be extrusion of the tissue destroyed by heat necrosis during ablation. In most cases, this complaint also typically resolved spontaneously within 6-8 weeks postoperatively. No active intervention was required. No cases of any vaginal infection were noted.
Discussion
This prospective study evaluates the use of microwave ablation for treating diffuse adenomyosis in a relatively large patient cohort. The results confirm the hypothesis that ultrasound-guided MWA can be a safe, effective, and minimally invasive option for women who wish to preserve their uterus while obtaining durable symptom relief. Compared with other uterine-sparing treatments such as UAE and HIFU, MWA offers several advantages (6,7. The precision of ultrasound guidance allows for targeted delivery of thermal energy, minimizing damage to surrounding tissues. Unlike UAE, which relies on embolic agents and induces ischemia in the entire uterine body, MWA focuses only on adenomyotic zones, preserving normal myometrial function. HIFU, while non-invasive, has limitations such as long treatment durations and dependence on favorable acoustic windows, which may not be available in all patients. The observed uterine volume reductions and symptom improvements are consistent with findings from smaller case series and pilot studies. Our data aligns with recent studies by Zhou et al. and Wang et al., where microwave ablation demonstrated significant pain and bleeding relief with a low rate of complications (7). Mechanistically, MWA causes tissue necrosis through rapid dielectric heating, creating uniform ablation zones that are less dependent on tissue impedance. This advantage may contribute to the consistently favorable outcomes seen in this study. The absence of serious complications supports its safety in clinical settings. Nonetheless, certain limitations must be acknowledged. First, the study lacked a control group, and long- term outcomes beyond 6 months remain unknown. Additionally, the absence of fertility-related outcomes limits conclusions for women desiring future pregnancies. A randomized controlled trial with extended follow-up would provide more robust evidence. Microwave ablation (MWA) induces tissue necrosis through dielectric heating: microwave-frequency electromagnetic waves cause rapid oscillation of polar molecules (primarily water) within tissues, generating frictional heat and resulting in coagulative necrosis of the cells (7,8). The MWA antenna is inserted directly into the target uterine tissue under imaging guidance, creating a localized electromagnetic field that heats the adenomyotic lesion from within. Unlike radiofrequency ablation, which is limited by rising tissue impedance as tissues desiccate and by heat-sink effects from blood flow (9), microwave energy can continue depositing thermal energy regardless of tissue charring and is less affected by perfusion. Consequently, MWA reaches higher temperatures more quickly and produces larger, more homogenous ablation volumes than radiofrequency methods, enabling more complete destruction of ectopic endometrial tissue. This mechanism is particularly advantageous for diffuse adenomyosis, as multiple or broad areas of the myometrium can be ablated in a controlled manner, reducing lesion volume and alleviating symptoms while preserving the overall uterine structure. Notably, MWA is a uterus-sparing approach—patients avoid hysterectomy—and clinical reports have shown significant symptom relief without impairment of ovarian function following MWA treatment for adenomyosis. Despite these therapeutic benefits, MWA is associated with certain adverse effects in gynecologic applications. Short-term post-ablation symptoms commonly include pelvic pain or cramping and vaginal bleeding or discharge as necrotic tissue is evacuated (9). Minor procedure-related injuries such as superficial skin burns at the probe entry site or transient nerve irritation have also been reported. Infection is a notable risk: if excessive thermal damage extends to or disrupts the endometrium, patients may develop endometritis or pelvic infection, and cases of post-ablation intrauterine infection have been documented. Longer-term effects include scar formation and pelvic adhesions – a particular concern in diffuse disease where large areas are ablated. Such adhesions can cause chronic pelvic pain or complicate future surgeries; for example, one case report described extensive post-MWA adhesions between the uterus and adjacent organs, which led to bowel obstruction, infection, and a urinary fistula after a subsequent hysterectomy. Although serious complications are rare, they can include unintended thermal injury to adjacent bowel or bladder (potentially causing perforation or fistula) as well as uterine perforation with hemorrhage. One clinical study (10) reported minor complications in 51.7% of cases and a ~5% incidence of major complications with MWA, underscoring that while the procedure is generally safe, meticulous technique and appropriate patient selection are critical to minimizing risks. Lastly, a note on pre-operative hormonal therapy. We have not used any pre-operative hormonal therapy in any of our cases. Some cases were referred to us from our Indian colleagues, wherein they were started on GnRH analogues or Norethisterone acetate (controlled release formulations) by the primary gynecologist to treat symptoms. However, we did not note any surgical differences while performing MWA on patients without pre-operative hormonal therapy when compared to patients with pre-operative hormonal therapy.
Conclusion
Microwave ablation appears to be a highly promising therapeutic modality for diffuse adenomyosis, offering significant symptom relief with minimal complications. Its uterus-sparing nature and real-time guidance make it an appealing alternative to hysterectomy for women with symptomatic disease. Our findings support the integration of MWA into the therapeutic algorithm for adenomyosis, especially in cases refractory to medical management. Further longitudinal studies are warranted to assess the durability of symptom control, the impact on fertility, and comparative efficacy with other interventional modalities.
References
Figure 1: Microwave Ablation probe with attachment cord. Note the insulated probe tip.
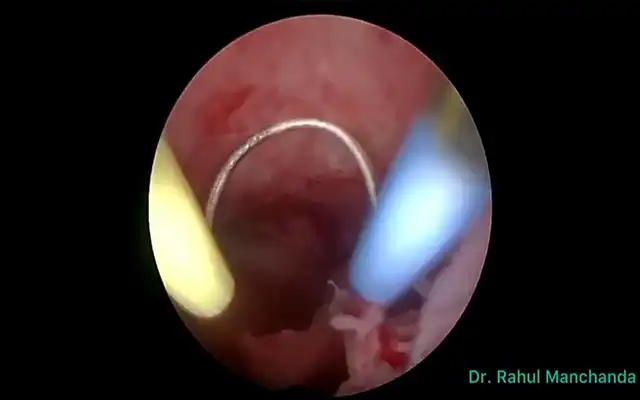
Figure 2: Intraoperative photo of microwave ablation probe during the procedure.
Figure 3: The Canyon KY 2000A MWA system. Note that the wattage is set at 100 Watts, the total time is at 7 minutes and the run time at the time of taking this picture is 6 minutes and 47 seconds. Probe temperature as recorded is 21.5 degrees Celsius.
Figure 4: VAS scores over time
Figure 5: PBAC scores over time
Figure 6: Uterine volume reduction with time