Authors / metadata
DOI : 10.36205/trocar3.2023005
Introduction
In recent years, HIFU therapy has become a viable surgical alternative for patients who still wish to have their uterus. However, adenomyosis is a disease that is very sensitive to the hormone estrogen, and HIFU therapy will not change the performance of hormones in the body. The risk of recurrence still exists. GnRH-A is a hormone that is commonly used for the treatment of adenomyosis, which can lower estrogen levels to menopausal levels and increase the atrophy of adenomyotic cells in the myometrium. This study provides several systematic reviews and meta-analyses of HIFU combined with GnRH Agonist (GnRH-A)
in adenomyosis and provides proof-based medical evidence for clinical applications.
Review
Adenomyosis is a gynecological disease characterized by ectopic endometrial tissue in the myometrium which often occurs in women during the reproductive age, between 30-40 years. The prevalence of adenomyosis currently ranges from 20-35%. The patient’s main clinical symptoms include abnormal uterine bleeding, menstrual pain (dysmenorrhea), and impaired fertility (infertility). The pathological mechanism for the occurrence of adenomyosis is an imbalance of steroid hormones, causing a local inflammatory process that is the cause of changes in cell proliferation which may lead to neuro-angiogenesis in myometrial tissue (1-4).
Current therapy for adenomyosis includes oral therapy, progesterone, contraceptive pills or anti-inflammatory medication as well as GnRH-A injections and adenomyomectomy that can be performed by minimally invasive laparoscopic surgery or laparotomic surgery (5).
Surgical action for the removal of the uterus (hysterectomy) is the main option for women who no longer want children, but hysterectomy for adenomyosis which occurs in infertile couples is not a good choice for women who still want children. Although Uterin Artery Embolization (UEA) treatment can improve patient symptoms, its effect on ovarian function and pregnancy are still uncertain (4,5).
High Intensity Focused Ultrasound (HIFU), an emerging non-invasive surgical technique for the treatment of benign tumors, has been used for adenomyosis since 2008. Under ultrasound or magnetic resonance (MRI) examination, HIFU energy can penetrate the abnormal target tissue and remove the lesion through thermal effects and cavitation and allows the preservation of normal tissue around the lesion. The cavitation process is a condition in which HIFU will create a predefined temperature raise in the targeted cells so that the liquid in the cells is heated until it becomes liquid vapor, which results in the formation of bubbles, filled with vapor in the liquid (6,7). The bubbles eventually explode and the released vapor penetrates into the surrounding tissues through a mechanism that initially softens and then gets absorbed by healthy body tissue.
The Criteria of Selection
The studies that are included in this meta- analysis do meet the following criteria: Vannuccini and Petraglia: this study compares HIFU combined with GnRH-A vs. HIFU solely in patients with adenomyosis (8,9). The HIFU group combined with GnRH-A is defined as the experimental group, the HIFU group itself is defined as the control group, the objects of the study are women aged 18–50 years, women with focal or diffuse adenomyosis diagnosed by ultrasound examination, MRI, or computed tomography (CT), patients who have not received any treatment for adenomyosis within three months prior to the study. Abbott Outcome indicators: The main outcome indicators are changes in uterine volume while adenomyotic lesions are defined as the main outcome. The secondary outcomes are the visual analog scale score (VAS) for dysmenorrhea, menstrual volume score, serum CA125 level, and recurrence rate.
The exclusion criteria are the following: animal experiments, case reports, conference abstracts, conference proceedings, editorial letters, guidelines or comments, repeated study, studies in which the full text is not available, patients with uterine fibroids or other gynecological diseases, whose clinical symptoms are similar to adenomyosis and study less than 3 months HIFU ablation (10).
Results
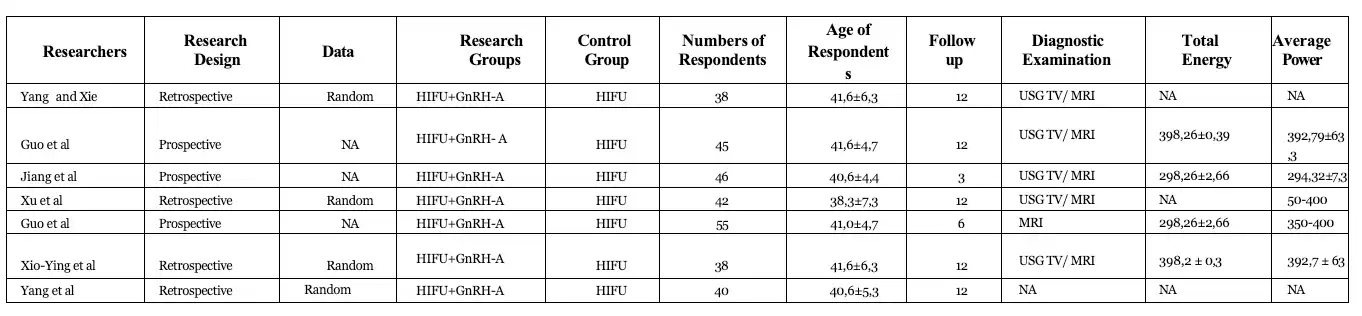
Of the 390 articles, 9 studies were obtained from data of 766 patients analyzed in this meta- analysis (11-19). Of the nine studies, one of them was using MRI for the imaging diagnosis of adenomyosis, six were using transvaginal ultrasound or MRI, and the two ones did not report specific imaging diagnostic methods. Although these studies provide information about the diagnostic imaging methods used, they do not provide specific imaging criteria for the diagnosis of adenoma.
Changes in the physiology of adenomyosis
Changes in uterine volume
Among the nine studies included, only three reported a method of generating random- location sequences, which was the random number table method. The analysis demonstrated the change of uterine volume as the rate of uterine volume reduction after HIFU in 232 patients. The results of the meta-analysis showed that the rate of uterine volume reduction in the HIFU group with GnRH-A was higher than that in the HIFU only group at 12 months after the procedure (13-20).
Changes in volume of adenomyotic lesions
Three studies (239 cases) reported changes in lesion size before and after HIFU ablation which showed that the volume of lesions in the experimental group was smaller than that in the control group in 3 and 6 months after the procedure. Although the results of the study showed no significant difference in VAS score for dysmenorrhea in both groups (p > 0.05). (11, 12, 17). A total of five studies (367 cases) used VAS to evaluate patients with dysmenorrhea. The results of the meta-analysis showed that the VAS score for dysmenorrhea in the HIFU group with GnRH-A was lower than the HIFU group alone after the procedure (11, 13, 14, 17, 18).
Menstrual Volume Score
Three studies (243 cases) used the menstrual volume score to evaluate menstrual bleeding. The results of the meta-analysis showed that the menstrual volume score of the HIFU group with GnRH-A was lower than that of the HIFU group itself after the procedure (14,16,19).
Levels of Serum CA125
Three studies (252 cases) evaluated patients’ levels of serum CA125. The results of the meta-analysis showed that serum CA125 levels in the HIFU group with GnRH-A were lower than the HIFU group alone after the procedure (11, 17, 18).
Recurrence Rate
Three studies (314 cases) compared the recurrence rates of the experimental and the control groups. The results of the meta-analysis showed that the relapse rate in the HIFU group with GnRH-A was lower than that in the HIFU group itself (15, 16, 19).
Pregnancy Outcome
One study reported patient pregnancy outcomes at 6 months after treatment. There were five pregnancies reported after the HIFU intervention combined with GnRH-A (n = 45), three of which delivered naturally and two ended in abortion. In the merely HIFU group (n = 46), there were four reported pregnancies following HIFU ablation, one resulting in natural delivery, one resulting in miscarriage and two ending in abortion (20).
Discussion
The results of this meta-analysis on the data from 766 patients showed that, HIFU combined with GnRH- A compared to mere HIFU group, for the treatment of adenomyosis had greater effectiveness in reducing uterine volume and adenomyotic lesions and alleviating symptoms.
Adenomyosis is a common and difficult gynecological disease that seriously affects women’s health and quality of life. Effective symptom relief, relapse prevention, and increased pregnancy rates are problems that have to be solved. Compared to currently available therapies, HIFU is a non- invasive and innovative technology for adenomyosis while still at risk of recurrence.
The therapeutic mechanism of HIFU produces thermal and cavitation effects causing the target tissue temperature at the focal point to rise above 60–100◦C, causing non-coagulation necrotic lesions. At the same time, the surrounding structures are not damaged. Previous studies found that uterine smooth muscle tissue in adenomyotic lesions was sensitive to HIFU. HIFU treatment was an effective and ideal treatment for adenomyosis. A retrospective study by Lee et al. enrolled 889 patients with adenomyosis who underwent ultrasound-guided HIFU (USgHIFU). The results revealed that the uterine volume reduction rate was 60.1% at 3, 6, and 12 months after the procedure, respectively. This was consistent with the results of a recent systematic and meta-analysis showing a substantial effect in reducing uterine volume after HIFU treatment for adenomyosis in 12 months (20).
GnRH-a therapy can effectively relieve pain in adenomyotic patients by reducing the regulation of GnRH receptors in the body, thereby reducing the level of gonadotropins secreted by the pituitary gland which results in decreased ovarian function.
HIFU combined with GnRH-A can help maintaining the effect of HIFU therapy and reduce relapse rates. Most of the studies involved, suggest that patients should be given GnRH-A three times after HIFU ablation. The first GnRH–A is given on the first to third day of the first menstruation after HIFU therapy. Then, the interval between the two GnRH-A injections is maintained at 28 days.
The results of the existing study show that the all symptoms of both groups are improved after the procedure, but the VAS or dysmenorrhea scores and menstrual volume scores in the HIFU group combined with GnRH-A are lower than in the mere HIFU group. The levels of serum CA125 are also decreased. Although the results of the VAS score for dysmenorrhea show that HIFU combined with GnRH-a can better alleviate dysmenorrhea in each patient, there is still
excessive heterogeneity. The relationship between adenomyosis and infertility is not clear, but adenomyosis can affect a woman’s fertility, this is mainly related to disruption and thickening of the myometrial junctional zone (JZ), and hypo acceptability of the endometrium. In recent years, due to the continuous improvement of various ultrasound diagnostic methods and the increasing age of women seeking infertility treatment, the rate of women with a diagnosis of adenomyosis among infertile women has increased. Traditionally, infertile patients with adenomyosis are treated with GnRH-A or they may have adenomyosis (adenomyomectomy) removed surgically. Studies have shown that HIFU is a safe and effective procedure for infertile women and it does not increase obstetric risk (21,22).
Conclusion
The results of this meta-analysis show that compared with mere HIFU treatment and HIFU accompanied by GnRH-A therapy performed on adenomyosis, obtained a greater level of effectiveness in reducing uterine volume the volume of adenomyotic lesions and alleviating symptoms. However, because the number of studies included is too small, further research that has a long- term evaluation is needed.