Authors / metadata
DOI: 10.36205/trocar5.2024001
Abstract
Introduction
Abnormal uterine bleeding is a real public health issue. The distribution of its causes varies from one setting to another. Hysteroscopy, which is the gold standard for structural causes of Abnormal uterine bleeding is not always accessible to our population and no local hysteroscopic data are available. The aim of this study was to determine the symptoms associated with hysteroscopic findings in a Congolese population in Kinshasa presenting with Abnormal uterine bleeding.
Methodology
This was a retrospective case series on 151 records of patients who underwent consecutive office hysteroscopy carried out by a single operator for Abnormal uterine bleeding between January 2018 and December 2022 in a private clinic in Kinshasa. Sociodemographic, clinical and hysteroscopic variables were retained. Descriptive statistics were used and comparison of proportions was made using Pearson’s Chi-square or Fisher’s Exact test. The test was significant at a p value less than 0.05.
Result
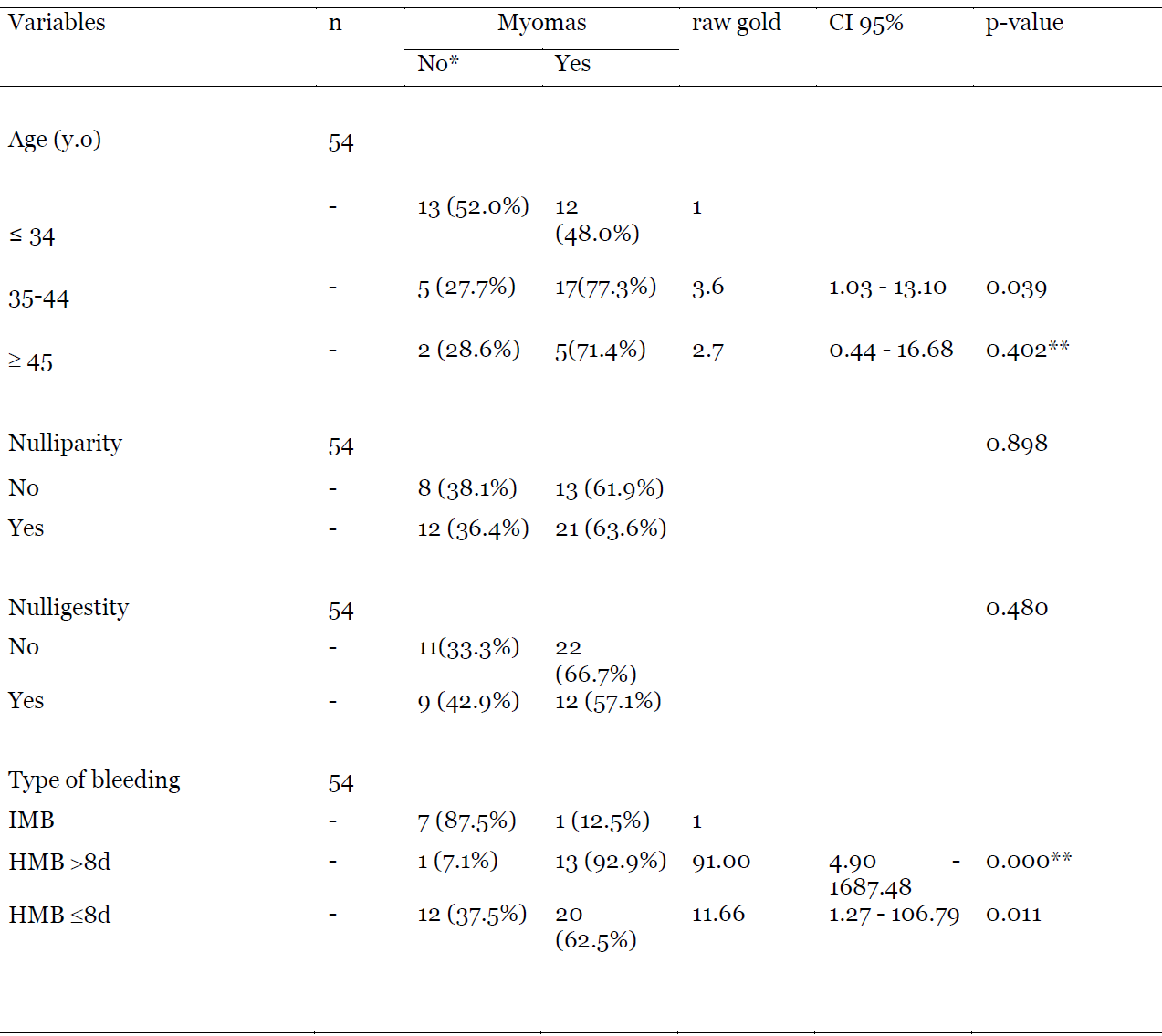
The mean age of patients was 37.53±10.89 years old. They were nulliparous at approximately 51% and the non-menopausal women represented 89.40%. Heavy menstrual bleeding less or equal to 8 days was the most frequent complaint (54.31%). The most common findings were endometrial polyps at 30.36%, uterine myomas at 23.81%, and adenomyosis at 8.93%. In 11.90% of cases, patients had no abnormality found on hysteroscopy. Heavy menstrual bleeding less or equal to 8 days and Heavy menstrual bleeding more than 8 days were symptoms associated with the discovery of myomas (respectively p = 0.011; OR = 11.66 and p = 0.000; OR = 91.00).
Conclusion
Endometrial polyps and uterine myomas are the most common structural causes of Abnormal uterine bleeding in our environment. This heavy bleeding mostly affects women aged 35 and over. Heavy menstrual bleeding less or equal to 8 days and Heavy menstrual bleeding more than 8 days are symptoms associated with myomas.
Introduction
Abnormal uterine bleeding (AUB), particularly excessive bleeding represents a major concern for patients throughout their lives, and therefore a public health issue [1]. Up to 30% of women will seek for help from a medical practitioner to counter this problem during their reproductive age [2]. It causes considerable health care burden on women, their families and society as a whole [3].
AUB significantly and negatively affects quality of life [4], and can have a negative economic impact due to the absenteeism in the workplace [5]. This bleeding can also be the result of serious pathologies such as endometrial cancer [6].
According to the FIGO Menstrual Disorders Working Group, the causes of AUB are summarized in the acronym PALM [Polyps-Adenomyosis-Leiomyomas-Malignancy] for structural causes and COEIN [Coagulopathy-Ovulatory dysfunctions-Endometrial causes-Iatrogenic causes-No otherwise classified] for non-structural causes [7].
Guin et al. [8] in 2011, in a study conducted in India, reported that in 30% of cases, endometrial hyperplasia was the most common hysteroscopic finding underlying AUB. While in China, in 2018, Sun et al. [9] found that endometrial polyps were the most common intrauterine condition in case of AUB, representing 16.2% of cases.
In Canada, Beaumont et al. [10] in 2000, found no abnormality on hysteroscopy in 76% of women with AUB while the pathological findings were dominated by endometrial polyps in 7% of cases. In Kenya, in 2020, Mutakha et al. [11] noted that uterine myomas were the most common intrauterine cause underlying AUB in 44.5% of cases.
The analysis of this great variability in the structural causes of AUB depending on the environment therefore prohibits the extrapolation of data from one environment to another. The research question was about the most common findings on hysteroscopy in Congolese women presenting with AUB. Considering the lack of local hysteroscopic studies in our setting, informations about local causes underlying structural AUB will help in developing management strategies, especially since in low-income countries, hysteroscopy, which is the gold standard for intrauterine pathologies, is not always accessible to most of women. It therefore seemed imperative to investigate the probability of different hysteroscopic findings in particular types of AUB in our environment. This was the aim of the present study. The objectives were to describe the sociodemographic and clinical characteristics of women with AUB, to describe hysteroscopic findings, and to identify symptoms associated with these hysteroscopic findings.
Material and methods
This was a retrospective case series of 151 records of patients who underwent consecutives hysteroscopies for AUB in an outpatient Clinic. They were received at Clinique d’Or in Kinshasa/Democratic Republic of Congo, from January the 1st, 2018 to December the 31st, 2022. All hysteroscopies were carried out by a single operator.
The variables retained were as follows: age, level of education, marital status, parity, gestational age, history of abortion/miscarriage,typeof abortion/miscarriage, menopausal status, type of bleeding, history of uterine surgery, type of surgery and hysteroscopic findings.
As a routine, before carrying out the hysteroscopy, explanations on the procedure, the possibility of performing certain procedures and the possible complications are provided to the patient. The procedure is routinely carried out without premedication and without anesthesia with psychological support provided by the team in the hysteroscopy room and particularly by the nurse.
Patients are placed in the gynecological position and procedures are performed using a 5 mm external diameter Bettocchi hysteroscope with a 2.9 mm 30-degree scope.
The uterine cavity is distended with 0.9% saline solution. Intrauterine pressure is obtained by gravity if visibility is satisfactory or by pressure on a soft infusion bag using a pneumatic chamber with a pressure gauge.
Data were entered with EPI DATA 3.1 and then analyzed with SPSS (Statistical Package for Social Sciences) 23.0 software.
The qualitative variables were displayed as proportions and the quantitative ones in mean and standard deviation or median and extreme depending on the case.
Comparison of proportions between groups was made using Pearson’s Chi-square or Fisher’s Exact test when appropriate. The Odds Ratio was used to measure the strength of association between variables.
The test was significant for a p value less than 0.05.
The present study obtained an approval from the Ethics Committee of the Kinshasa School of Public Health (ESP/CE/134/2022).
Results
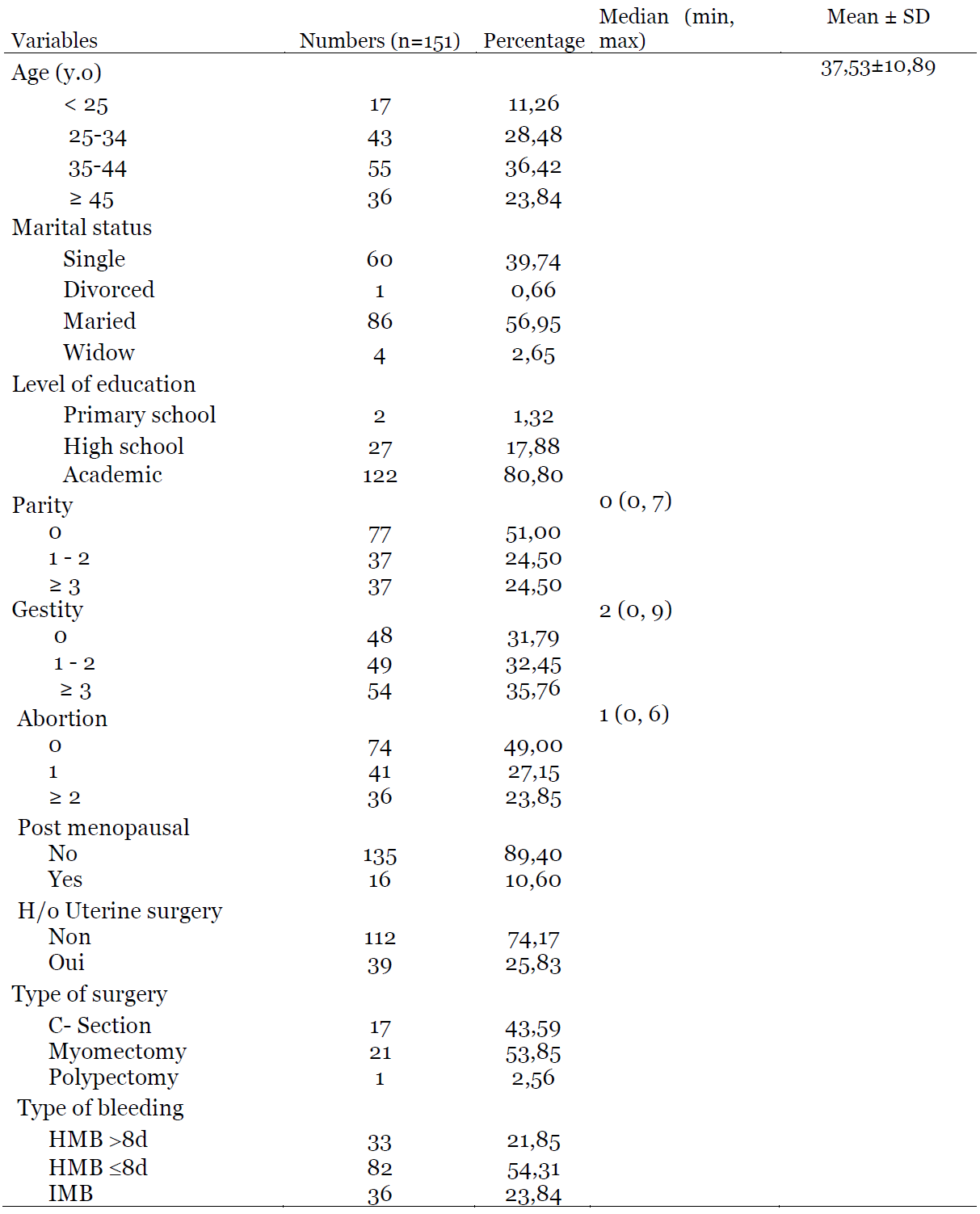
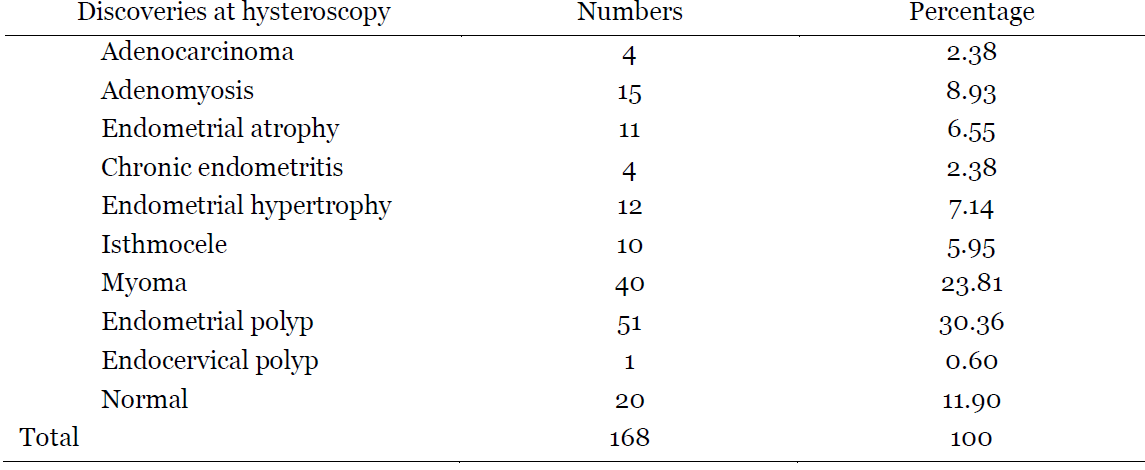
The mean age of patients was 37.53±10.89 years old. More than a half was married (56.95%) and more than three quarters (80.80%) had a university level. The majority was nulliparous (51%) while almost a quarter (24.50%) was multiparous. Patients who had never achieved a pregnancy represented 31.79% while those who had experienced at least one abortion represented 51%. Premenopausal patients constituted more than three quarters (89.40%) of the present study. In terms of surgical history, 25.83% of patients had a uterine surgery. More than a half (54%) of these patients had undergone a myomectomy while cesarean section represented 44%. HMB ≤8d was the most reported AUB (54.31%), followed by intermenstrual bleeding (IMB) (23.84%). (Table 1). Endometrial polyps and myomas were the most frequently encountered pathologies in hysteroscopy, with 30.36% and 23.81% respectively. Adenocarcinoma was found in 2.38% of cases. In the presence of AUB, up to 11.90% of patients did not present any abnormality on hysteroscopy. (Table 2).
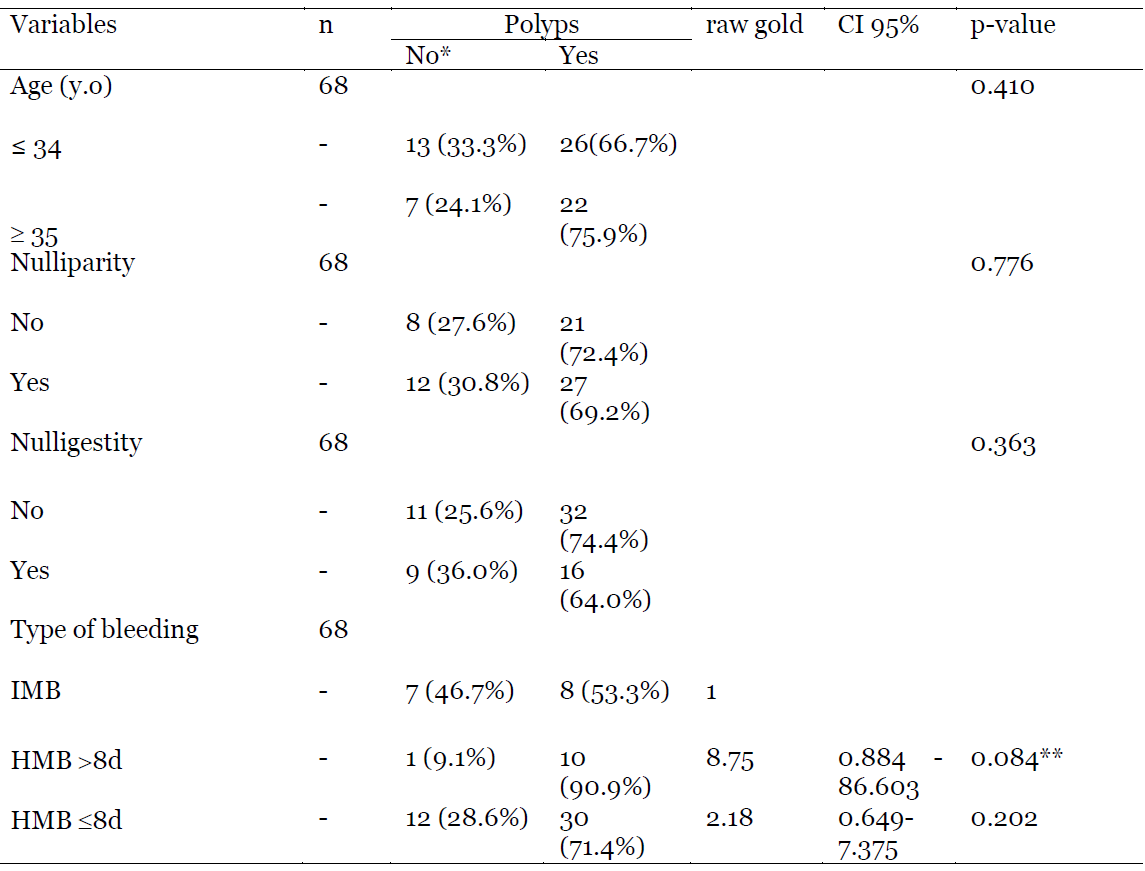
Regarding the factors associated with polyps as hysteroscopic findings, Table 3 shows that there was no difference in the finding of polyps in patients based on age, nulliparity, nulligestity and the type of bleeding. (p respectively of 0.410; 0.776; 0.363; 0.084 and 0.202).
Compared to women under 34 years old, those from 35 to 44 years old had 4 times the risk of having myomas (OR = 3.6; 95% CI 1.03 – 13.10) in hysteroscopy when presenting with AUB. Considering the type of bleeding, patients presenting with HMB >8d, had 91 times more risk of having myomas compared to those who had HMB ≤8d (OR = 91.00; 95% CI 4.90 – 1687.48). This risk was 12 times when taking in account HMB ≤8d (OR = 11.66; 95% CI 1.27 – 106.79). Nulliparity and nulligestity were not associated with the finding of myomas (p-value of 0.898 and 0.480 respectively). (Table 4).
Discussion
In the present study, the mean age of patients was 37.53±10.89 years old. This finding is in agreement with those made in China and Canada by Sun et al. [9] and Beaumont et al. [10](respectively 35.9 and 34.3 years). Thisage is therefore within the range of thoseadvanced in the literature, very close to thepre-menopausal period [12,13]. Indeed,menstrual disorders constitute one of themost frequent complaints among women inperimenopausal period. The latter integratesinto its definition several parametersincluding those linked to age, physiologicaland biological changes and alsosymptomatology [14].
With regard to age, certain pathologies which are responsible for AUB, in particular polyps and uterine myomas, have a symptomatology which peaks between the thirties and the forties due to the disturbances in the functioning of the hypothalamic-pituitary-ovarian axis, frequent during this period [14].
Regarding reproductive characteristics, nulliparous women represented 51% in the present study. This proportion is close to that found by Ghimire et al. [15] which was 61%. However, it differs from the larger proportions of multiparous women reported by Pandey et al. [16], Kashyap et al. [17] and Vijayan et al. [18].
Indeed, in the present study and in that conducted by Ghimire et al. [15], polyps and myomas were the most frequent hysteroscopic findings associated with AUB. Several studies report the existence of an inverse association between parity and the occurrence of myomas and polyps, suggesting a protective effect of multiparity. Myomas and polyps are twice as common in nulliparous women compared to others [19,20]. These myomas can lead to AUB by increasing the endometrial surface area associated with weakening and engorgement of vessels [21], and by supplanting platelet action due to the increase in vascular flow in the areas presenting lesions [22]. In addition, polyps and myomas can be responsible for female infertility, which could explain the preponderance of nulliparity. Several pathophysiological mechanisms have been put forward, in particular by proximal tubal occlusion, modification of gamete transport, deformation of the uterine cavity, abnormal contractility with modification of endometrial vascularization and finally the reduction of endometrial receptivity. This reduction is consecutive to an alteration of the implantation mechanisms by inflammatory phenomena and a reduction in the level of Homeobox (Hox) of the HOXA-10 and HOXA-11 type [23-27].
The discrepancy of the data of the present study compared to those of Pandey et al. [16], Kashyap et al. [17] and Vijayan et al. [18] is probably due to the fact that the presence of these structural anomalies does not necessarily have an impact on a woman’s fertility because many of them conceive and give birth with myomas and polyps [27].
Regarding the type of AUB, HMB ≤8d was the most reported complaint. This finding is similar to those noted by Vijayan et al. [17] and Radhikabai et al. [28] who reported frequencies of 76.1% and 31.25% respectively.
The finding of the present study is in agreement with the literature data which places HMB ≤8d in first position as the most reported complaint in case of AUB [29]. Several studies report polyps and myomas as the most common structural pathologies in cases of AUB [28,30]. These can lead to AUB, particularly HMB through mechanisms previously mentioned.
In this study, endometrial polyps were the most common finding at approximately 30.36% and patients presented indifferently with HMB ≤8d, IMB and HMB >8d. This proportion is similar to those found by Lasmar et al. [31], Malik et al. [32] and Ghimire et al. [15] which were respectively 33.9%; 30% and 46.67%. It diverges from those noted by Kaur et al. [33] and Guin et al. [8]who reported endometrial hyperplasia asthe most common finding in 30% of cases. Inthese last two studies, most of patients weremultiparous. These studies, mainly carriedout in India, a country with a high populationdensity [34] where laws on birth control arerestrictive. This could justify the use ofcontraceptive methods, including estrogentherapy that is not or only minimallycounterbalanced [35] being a risk factorunderlying endometrial hyperplasia. Butalso, the high prevalence of obesity andmetabolic syndrome in India [36] couldexplain this high frequency of endometrialhyperplasia, due to the aromatization inadipose tissue of delta-4 Androstenedioneand testosterone into estrone and estradiolrespectively [37-38].
Myomas represented the second most common pathology in approximately 24%. This proportion is close to that of 29 % found by Kumari et al. [39] whose study focused on peri-menopausal women who had AUB. A longitudinal study estimated that the risk of developing fibroids in a woman over 45 years of age is greater than 60% with a higher incidence during peri-menopause [19]. Indeed, in the present study, 60% of the patients were aged at least 35 years. A similar observation was also observed by Kumari et al. [39] who reported a proportion of approximately 66% of women aged from 40 to 45 years in the perimenopausal period. The essential characteristic of these patients is reflected by a dysfunction of the hypothalamic-pituitary-ovarian axis leading to a climate of relative hyperestrogenism [40].
It has been clearly recognized for several years the role of hormones (estrogens and progesterone) in the occurrence of myomas [41]. In fact, estrogens have a well-accepted mitotic effect, mediated largely by growth factors and by autocrine and paracrine regulation. Estradiol (E2) experimentally stimulates the growth of uterine smooth muscle cells [41]. Although plasma estradiol levels are not necessarily high, we recognize the essential role of a local hyper estrogenic environment (higher concentrations of E2, estrone and their sulfates). It results from metabolic abnormalities, such as reduced conversion of E2 to estrone, and higher concentrations of cytochrome P450 (aromatase) [41].
Endometrial cancer was found in 2.38% of cases in the present study. This proportion seems to be close to the lifetime risk of presenting endometrial cancer, which is approximately 2.6% [42], and diverges from those found by Giannella et al. [43] and Saccardi et al. [44].
In fact, the frequency of 2.38% reported in this study is high compared to 1.3% found by Giannella et al. [43]. It represents approximately one tenth (21%) of that reported by Saccardi et al. [44]. This may be due to the fact that, in the present study, approximately 11% of patients were postmenopausal and endometrial biopsy was only done when there were macroscopically suspicious lesions. Giannella et al. working on the prediction of endometrial hyperplasia and cancer in pre-menopausal women with AUB had excluded postmenopausal women, who are at greatest risk of developing endometrial cancers [45-46]. On the other hand, the study by Saccardi et al. [44] which focused on predicting the risk of endometrial cancer based on the indication for diagnostic hysteroscopy was carried out on women in peri-menopause or menopause with a systematic endometrial biopsy, which could maximize the probability of finding cases of endometrial cancer.
Limitations of the study
The present study borders on having been a documentary retrospective. Therefore, it did not cover all possible variables in patients with. Anamnestic elements leading, for example, to non-structural causes could not be identified while a significant fraction of patients with AUB had no structural lesion on hysteroscopy. The non-systematic performance of histopathology and the lack of information about the economic level of the patients are others limitations linked to the retrospective nature of the present study.
Conclusion
In this study, endometrial polyps and myomas were the most common findings at hysteroscopy in the presence of AUB. HMB ≤8d and HMB >8d which are the main manifestations, are associated symptoms in the discovery of polyps and myomas in women aged 35 and over. Thus, for our environment where hysteroscopy is not accessible to many patients, in any case of AUB, we must raise polyps and myomas as the first hypotheses and systematically search them using ultrasound which is an available tool.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Mindombe Moleko Patrick: participation in design, statistical analyzes and writing.
Lumingu Lusakueno Armand: participation in the design.
Biawila Lusila Bruno: participation inthe design.
Kusuman Amos: participation indesign and writing.
Odimba Mpoy Jules: participation inthe design.
Kintoki Makundika Olivier: participation in the design.
Ndesanzim Otem Christian: participation in the design.
Nzau-Ngoma Emmanuel: researchdesign, statistical analysis andvalidation of the final version of themanuscript.
References
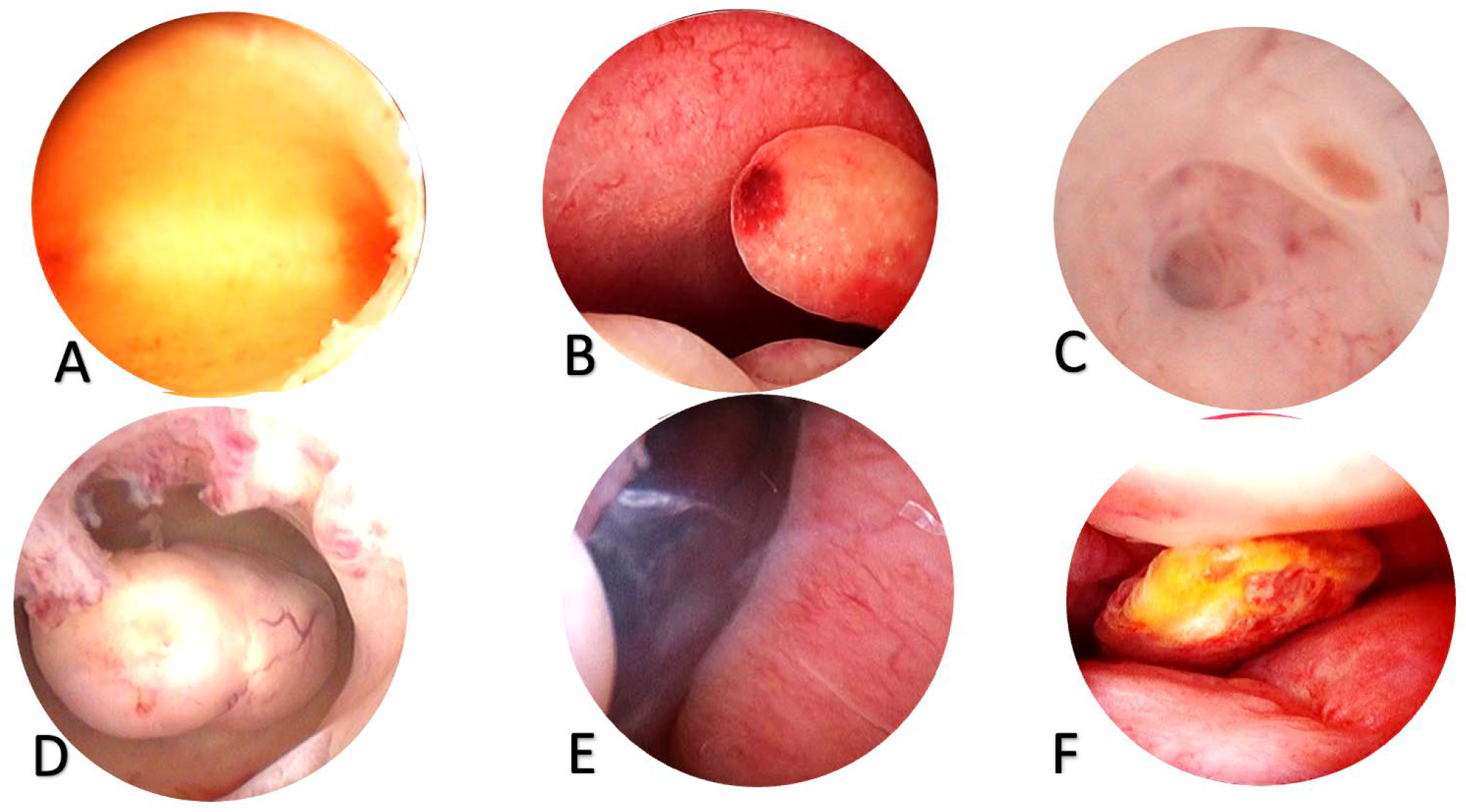
Figure 1. Findings in hysteroscopy
A.Negative hysteroscopic view, B. Endometrial polyps, C. Adenomyosis (we see a nest and a holeleading into the depth of the myometrium, D. Uterine myoma, E. Hypertrophy of the endometrium with a polypoid appearance of the endometrium, F. Adenocarcinoma of the endometrium (we have a polypoid mass with an area of necrosis).
Table 1: Sociodemographic and clinical characteristics of women with AUB
Table 2: Diagnosis made at hysteroscopy in patients with AUB
Table 3: Association between Polyps and some variables
Table 4: Association between myomas and some variables